Geology and Mineralogy This Document Consists of 19 Pages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
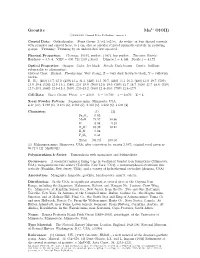
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

GUIDE MINERALS and ROCKS
GUIDE to the MINERALS and ROCKS of Minnesota by GEORGE M. SCHWARTZ and GEORGE A. THIEL DEPARTMENT OF GEOLOGY and MINNESOTA GEOLOGICAL SURVEY UNIVERSITY OF MINNESOTA Guide to the Minerals and Rocks of Minnesota by G. M. Schwartz, Director, Minnesota Geological Survey George A. Thiel, Chairman, Department of Geology University of Minnesota Introduction The minerals and rocks of Minnesota serve as the basis of many im portant industries. They supply the parent materials for our many different types of soils; they contain our vital underground water supplies; and they furnish the raw materials for extensive mineral industries. Many people are interested in the rocks and minerals which they find in road-cuts, in excavations, on their farms, and on their vacation trips. Unusual specimens arouse the curiosity of the finder. Some specimens have peculiar shapes, some have attractive colors and others appear to contain ores of economic value: Many such specimens are received each year at the Department of Geology and the Minnesota Geological Survey of the University of Minnesota. The specimens are generally accompanied by a request for information in regard to the composition, manner of formation, and commercial value of the rock or mineral involved. In response to an increasing number of inquiries from individuals and from schools, this pamphlet was prepared to summarize, as far as possible in nontechnical terms, the different kinds of rocks and minerals found in Minnesota and to describe them so that the amateur collector, the school teacher, the boy and girl scout and other interested persons can identify them. In a pamphlet of this size it is impossible to outline in detail the almost infinite varieties of the common rocks and minerals. -

Minerals of Arizona Report
MINERALS OF ARIZONA by Frederic W. Galbraith and Daniel J. Brennan THE ARIZONA BUREAU OF MINES Price One Dollar Free to Residents of Arizona Bulletin 181 1970 THE UNIVERSITY OF ARIZONA TUCSON TABLE OF CONT'ENTS EIements .___ 1 FOREWORD Sulfides ._______________________ 9 As a service about mineral matters in Arizona, the Arizona Bureau Sulfosalts ._. .___ __ 22 of Mines, University of Arizona, is pleased to reprint the long-standing booklet on MINERALS OF ARIZONA. This basic journal was issued originally in 1941, under the authorship of Dr. Frederic W. Galbraith, as Simple Oxides .. 26 a bulletin of the Arizona Bureau of Mines. It has moved through several editions and, in some later printings, it was authored jointly by Dr. Gal Oxides Containing Uranium, Thorium, Zirconium .. .... 34 braith and Dr. Daniel J. Brennan. It now is being released in its Fourth Edition as Bulletin 181, Arizona Bureau of Mines. Hydroxides .. .. 35 The comprehensive coverage of mineral information contained in the bulletin should serve to give notable and continuing benefits to laymen as well as to professional scientists of Arizona. Multiple Oxides 37 J. D. Forrester, Director Arizona Bureau of Mines Multiple Oxides Containing Columbium, February 2, 1970 Tantaum, Titanium .. .. .. 40 Halides .. .. __ ____ _________ __ __ 41 Carbonates, Nitrates, Borates .. .... .. 45 Sulfates, Chromates, Tellurites .. .. .. __ .._.. __ 57 Phosphates, Arsenates, Vanadates, Antimonates .._ 68 First Edition (Bulletin 149) July 1, 1941 Vanadium Oxysalts ...... .......... 76 Second Edition, Revised (Bulletin 153) April, 1947 Third Edition, Revised 1959; Second Printing 1966 Fourth Edition (Bulletin 181) February, 1970 Tungstates, Molybdates.. _. .. .. .. 79 Silicates ... -

The Crystal Structure of Ramsdellite from Pirika Mine
Title The Crystal Structure of Ramsdellite from Pirika Mine Author(s) Miura, Hiroyuki; Kudou, Haruhiko; Choi, Jai Ho; Hariya, Yu Citation 北海道大学理学部紀要, 22(4), 611-617 Issue Date 1990-08 Doc URL http://hdl.handle.net/2115/36769 Type bulletin (article) File Information 22_4_p611-617.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Jour. Fac. SeL, Hokkaido Univ., Ser. IV, vol. 22, no. 4, Aug., 1990, pp. 611 -61 7. THE CRYSTAL STRUCTURE OF RAMSDELLITE FROM PIRIKA MINE by Hiroyuki Miura, Haruhiko Kudou: Jai Ho Choi and Yu Hariya (with 3 text-figures and 4 tables) Abstract Chemical composition and crystal structure of ramsdellite from Pirika mine, Hokkaido, Japan were studied. Chemical composition, determined by EPMA, shows that ramsdellite is pure manganese oxide and does not contain any other element. The crystallographic data, obtained by Rietveld analysis, indicate that ramsdellite has orthorhombic symmetry. Space group is Pnma and unit cell parameters are: a = 4.513Cl) A, b = 9.264 Cl) A and c = 2.859Cl)A . The structure consists of MnOs double chains CBystrom, 1950). There are 4 symmetry-dependent MnOs octahe dra in an unit cell and 2 symmetry- independent oxygen atoms in an MnOs octahedron. The difference of inter-atomic distances between Mn-OCl) and Mn -O(2) is larger than other manga nese dioxide minerals. The distortion of MnOs octahedron is quite similar to that of groutite Ca - MnOOH). These data suggest that ramsdellite was formed by oxidation of groutite. Introduction There are several kinds of manganese dioxide minerals such as pyrolusite, hoi· landite, cryptomelane, nsutite, birnessite and ramsdellite. -

Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan
Title Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan Author(s) Hariya, Yu Citation Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and mineralogy, 10(4), 641-702 Issue Date 1961-03 Doc URL http://hdl.handle.net/2115/35921 Type bulletin (article) File Information 10(4)_641-702.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP MKNERALOGICAL STCIDXES ON MANGANESE g]bXCbXgDE ,AND HYDROXIDE MENERALS gN ffE(OKKAI'DO, JAPAN By Yku IEIARIyA (With 29 Text Figures, 17 Tables and 3 Plates) Contribution from the Department of Geology and Mineralogy, Faculty of Seience, Hokkaido University No. 825 CONTENTS I. Introduction,.,..,,.,,.,,.,,,,.,.,,...,,,...,,.,.,,.,.,.,,,.,,., 642 II. Geoteetonic constitution of the Island of Hokkaido ,.,..,.,,.,... 643 1. Geographieal diseribution of mang4nese deposits in Hokkaido . , 644 A. South-western Tegion .,..,.,.,.,....,,.....,..,,,,,.....,.. 644 1) Western sub-province ..,.,.,.,....,,.,,...,.,,..,..,..... 644 a) Palaeozoic deposits .,..,.,.,...,..,..,,.,.,.,,,,,..,.,.,. 645 b) Neogene Tertiary deposits .,,,.,,.,...,.,.,...,.,,.,.,., 645 2) Eastern sub-province.,..,.,.,.,...,,..,,...,.,.,,,..,.,.,. 646 B. Axia} zone.,.,,.,..,.,.,..,...,,,.....,..,,,....,....,...... 646 C. North-eastern region,.,,,.,..,,,,.,.,,,.,,...,.,,,,.,.,.,.,, 647 D. Quaternary volcanie zones .,.,.,,,.,...,..,.,..,.,,.,..,.-・・ 647 2. 0n the respeetive manganese minerals of the above described deposits ......,..,..,..,.,,...,.,.,,,,,..,.,.,.,..,..,....... -

Coupled Substitutions in Natural Mno(OH) Polymorphs: Infrared Spectroscopic Investigation
minerals Article Coupled Substitutions in Natural MnO(OH) Polymorphs: Infrared Spectroscopic Investigation Nikita V. Chukanov 1,2,*, Dmitry A. Varlamov 3 , Igor V. Pekov 2, Natalia V. Zubkova 2, Anatoly V. Kasatkin 4 and Sergey N. Britvin 5 1 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow Region, Russia 2 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia; [email protected] (I.V.P.); [email protected] (N.V.Z.) 3 Institute of Experimental Mineralogy Russian Academy of Sciences, 142432 Chernogolovka, Russia; [email protected] 4 Fersman Mineralogical Museum of the Russian Academy of Sciences, Leninsky Prospekt 18-2, 119071 Moscow, Russia; [email protected] 5 Crystallography Department, Institute of Earth Sciences, St. Petersburg State University, University emb. 7/9, 199034 St. Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Solid solutions involving natural Mn3+O(OH) polymorphs, groutite, manganite, and feitknechtite are characterized and discussed based on original and literature data on the chemical composition, powder and single-crystal X-ray diffraction, and middle-range IR absorption spectra of these minerals. It is shown that manganite forms two kinds of solid-solution series, in which 4+ 3+ intermediate members have the general formulae (i) (Mn , Mn )O(OH,O), with pyrolusite as the 4+ 3+ 2+ Mn O2 end-member, and (ii) (Mn ,M )O(OH, H2O), where M = Mn or Zn. In Zn-substituted 2+ 3+ Citation: Chukanov, N.V.; Varlamov, manganite from Kapova Cave, South Urals, Russia, the Zn :Mn ratio reaches 1:1 (the substitution 3+ 2+ − D.A.; Pekov, I.V.; Zubkova, N.V.; of Mn with Zn is accompanied by the coupled substitution of OH with H2O). -

Descriptions of Northeast Asia Metallogenic Belts
Northeast Asia Metallogenc Belt Descriptions – May 5, 2004 DESCRIPTIONS OF NORTHEAST ASIA METALLOGENIC BELTS By Sergey M. Rodionov1, Alexander A. Obolenskiy2, Elimir G. Distanov2, Gombosuren Badarch3, Gunchin Dejidmaa4, Duk Hwan Hwang5, Alexander I.Khanchuk6, Masatsugu Ogasawara7, Warren J. Nokleberg8, Leonid M. Parfenov9, Andrei V. Prokopiev9, Zhan V. Seminskiy10, Alexander P. Smelov9, Hongquan Yan11, Yuriy V. V. Davydov9, Valeriy Yu. Fridovskiy12 , Gennandiy N. Gamyanin9, Ochir Gerel13, Alexei V. Kostin9, Sergey A. Letunov14, Xujun Li11, Valeriy M. Nikitin12, Vladimir V. Ratkin6, Vladimir I. Shpikerman15, Sadahisa Sudo7, Vitaly I. Sotnikov2, Alexander V. Spiridonov14, Vitaly A. Stepanov16, Fengyue Sun11, Jiapeng Sun11, Weizhi Sun11, Valeriy M. Supletsov9, Vladimir F. Timofeev9, Oleg A. Tyan9, Valeriy G. Vetluzhskikh9, Koji Wakita7, Yakov V. Yakovlev9, and 14 Lydia M. Zorina Edited by Sergey M. Rodionov1, Alexander A. Obolenskiy2, Zhan V. Seminskiy10, Tatiana V. Bounaeva14, and Warren J. Nokleberg8 1 Russian Academy of Sciences, Khabarovsk 2 Russian Academy of Sciences, Novosibirsk 3 Mongolian Academy of Sciences, Ulaanbaatar 4 Mineral Resources Authority of Mongolia, Ulaanbaatar 5 Korean Institute of Geology, Mining, and Mineral Resources, Taejon 6 Russian Academy of Sciences, Vladivostok 7 Geological Survey of Japan/AIST, Tsukuba 8 U.S. Geological Survey, Menlo Park 9 Russian Academy of Sciences, Yakutsk 10 Irkutsk State Technical University, Irkutsk 11 Jilin University, Changchun 12 Yakutian State University, Yakutsk 13 Mongolian University of Science and Technology, Ulaanbaatar 14 Russian Academy of Sciences, Irkutsk 15 Russian Academy of Sciences, Magadan 16 Russian Academy of Sciences, Blagoveschensk 1 Northeast Asia Metallogenc Belt Descriptions – May 5, 2004 Introduction and Companion Studies The metallogenic belts of Northeast Asia are herein synthesized, compiled, described, and interpreted with the use of modern concepts of plate tectonics, terranes and overlap assemblages, and mineral deposit models. -

Merumite a Complex Assemblage of Chromium Minerals from Guyana
Merumite A Complex Assemblage of Chromium Minerals from Guyana GEOLOGICAL SURVEY PROFESSIONAL PAPER 887 Prepared in cooperation with the Geological Survey of Guyana MERUMITE A COMPLEX ASSEMBLAGE OF CHROMIUM MINERALS FROM GUYANA r 0 1 mm 0.1 mm 0.1 mm 0.1 mm Photomicrographs of four merumite thin sections illustrating the diversity of mineral composition and structure and the fineness of grain size. Plain transmitted light. UPPER LEFT: Green eskolaite and red-brown guyanaite. UPPER RIGHT: Banded structure of green eskolaite and yellow to brown guyanaite. The very bright polygonal area is a cross section of a quartz crystal. LOWER LEFT : Reddish grimaldiite-mcconnellite aggregate filling LOWER RIGHT : Green eskolaite and yellow-brown guyanaite opening in merumite specimen. showing banded structure. Merumite A Complex Assemblage of Chromium Minerals from Guyana By CHARLES MILTON, D. E. APPLEMAN, M. H. APPLEMAN, E. C. T. CHAD, FRANK CUTTITTA, J. I. DINNIN, E. J. DWORNIK, B. L. INGRAM, and H. J. ROSE, JR. GEOLOGICAL SURVEY PROFESSIONAL PAPER 887 Prepared in cooperation with the Geological Survey of Guyana UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress Cataloging in Publication Data Main entry under title: Merumite a complex assemblage of chromium minerals from Guyana. (Geological Survey professional paper 887) Bibliography: p. Supt. of Docs, no.: I 19.16:887 1. Merumite Guyana. I. Milton, Charles, 1896- II. Series: United States. Geological Survey. Professional paper 887. QE391.M45M47 549'.7 74-16247 For sale by the Superintendent of Documents, U.S. -

Groutite, Hmno2, a New Mineral of the Diaspore-Goethite Group
GROUTITE, HMNO2, A NEW MINERAL OF THE DIASPORE-GOETHITE GROUP JouN W. GnuNnn, (Jniaer sity oJ M innesota, M inneapolis, M innesota. Arsrnacr Groutite, HMnO2 is a new member of the diaspore-goethite group. It resembles man- ganite in general appearance as in luster, color, streak, hardness and specific gravity. Its structure, crystal forms and habits, cleavage and pleochroism are different. It occurs on the Cuyuna range, Minnesota, associated with irori ores. INrnooucrroN Tn 1942the writer was presentedwith a small group of beautiful black crystals of a manganesemineral which had been thought to be manganite. The crystal habit of the mineral was so different, however, that an x-ray powder diagram was prepared which gave a pattern entirely difierent from any other known manganesemineral. A search for more material revealed that the mineral is not rare in the iron mines of the Cuyuna range of Minnesota. Somevery fine specimenswere presentedto the writ- er by Mr. GeorgeChamberlin, chief mining engineerof Pickands, Mather and Company at Crosby, Minn. The original sample examined was ob- tained from Mr. Harvey J. Hakala, mining engineer of the Oliver Iron Mining Company. It came from the Sagamoreopen pit west of Ironton on the Cuyuna range. Dr. R. B. Ellestad made the analysis. To all thesemen the writer is greatly indebted. Grants by the Graduate School of the University of Minnesota are gratefully acknowledged. CnvsraltocRAPHY Very beautiful specimens of hundreds of crystals, up to 5 mm. in greatest dimensions were available. Examination shows that the vast majority of the crystals are wedge or lens-shapedand that their faces are rounded and curved in a way that makes their measurementsalmost im- possible. -

Neutron and Synchrotron X-Ray Diffraction Study of the Structures and Dehydration Behaviors of Ramsdellite and “Groutellite”
American Mineralogist, Volume 89, pages 969–975, 2004 Neutron and synchrotron X-ray diffraction study of the structures and dehydration behaviors of ramsdellite and “groutellite” JEFFREY E. POST1,* AND PETER J. HEANEY2 1Department of Mineral Sciences, Smithsonian Institution, Washington, D.C. 20560-0119, U.S.A. 2 Department of Geosciences, 309 Deike, Pennsylvania State University, University Park, Pennsylvania 16802, U.S.A. ABSTRACT The crystal structure of ramsdellite, MnO2, was refined using time-of-flight powder neutron dif- fraction data and the Rietveld method in order to assess the effects of reduction in cathodic battery materials. For the first time, we present a refined structure for “groutellite,” a heretofore poorly char- 4+ 3+ acterized phase with ideal formula (Mn0.5Mn0.5)O1.5(OH)0.5. “Groutellite” is generated synthetically as an intermediate compound during the reduction of ramsdellite to groutite (MnOOH), and it also occurs as an intergrowth in certain natural specimens of ramsdellite. The Jahn-Teller distortions in “groutellite” are confined to thea -c plane, and they result in a 6.8% unit-cell volume increase relative to ramsdellite. The Mn–O bond lengths refined for “groutellite” are consistent with the replacement of half of the Mn4+ and O2- in ramsdellite by Mn3+ and (OH)–, respectively. In addition, the high- temperature behaviors of ramsdellite and “groutellite” were investigated by temperature-resolved synchrotron powder X-ray diffraction from 298 to 720 K. Rietveld refinements revealed a gradual thermal expansion of the groutellite structure to ∼450 K. At higher temperatures, the unit-cell volume gradually decreased, primarily as a result of a decrease in c. -

Guide to Mineral Collecting in Minnesota
UNIVERSITY OF MINNESOTA 1974 Minnesota Geological Survey Matt Walton, Director St. Paul, Minnesota 55108 Guide to Mineral Collecting in Minnesota G. R. Rapp, Jr. and D. T. Wallace Department of Geology and Geophysics University of Minnesota Illustrations by Ann Cross WATEII fEATURES AND DRAINAGE BASINS or NI.NESOT' WISCONSIN IOWA ineral collecting appeals to more than six million M Americans. Rocks and minerals provide many clues to what we know about nature. Our knowledge of the age of the earth, the nature of prehistoric life, and the record of the great ice ages comes from what we can determine from the study of rock strata. This booklet is about rocks and minerals in Minne sota. It is intended for the general public, particu larly for those individuals that are just awakening to or are renewing an earlier interest in rocks. We hope to point the beginner in a direction that will provide an interesting and rewarding hobby. To do this we offer some essential background on rocks and minerals and a detailed guide to many of Minnesota's more attractive rock and mineral specimens. Although the terms minerals and rocks are often used interchangeably, it is not entirely correct to do so. Minerals are distinct chemical species. Each mineral has its own chemical formula and crystal structure. Indeed, minerals are often referred to as crystals, especially if they exhibit crystal faces. Rocks, on the other hand, are aggregates of several minerals. Just as minerals are composed of certain specified elements, each rock type is composed of a certain group of minerals. -

Petrology, Structural Geology, and Significance of Mn-Andalusite from the Lower Ortega Quartzite, Tusas MTS., NM, USA Nancy A
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2007 Petrology, Structural Geology, and Significance of Mn-Andalusite from the Lower Ortega Quartzite, Tusas MTS., NM, USA Nancy A. Price University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Part of the Geology Commons, and the Other Earth Sciences Commons Price, Nancy A., "Petrology, Structural Geology, and Significance of Mn-Andalusite from the Lower Ortega Quartzite, Tusas MTS., NM, USA" (2007). Masters Theses 1911 - February 2014. 2578. Retrieved from https://scholarworks.umass.edu/theses/2578 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. PETROLOGY, STRUCTURAL GEOLOGY, AND SIGNIFICANCE OF MN-ANDALUSITE FROM THE LOWER ORTEGA QUARTZITE, TUSAS MTS., NM, USA A Thesis Presented by NANCY A. PRICE Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of MASTERS OF SCIENCE August 2007 Geosciences PETROLOGY, STRUCTURAL GEOLOGY, AND SIGNIFICANCE OF MN-ANDALUSITE FROM THE LOWER ORTEGA QUARTZITE, TUSAS MTS., NM, USA A Thesis Presented by Nancy A. Price Approved as to style and content by: Michael L. Williams, Chair Michael J. Jercinovic. Member Sheila Seaman, Member Michael L. Williams, Department Head Department of Geosciences ABSTRACT PETROLOGY, STRUCTURAL GEOLOGY, AND SIGNIFICANCE OF MN-ANDALUSITE FROM THE LOWER ORTEGA QUARTZITE, TUSAS MTS., NM, USA AUGUST 2007 NANCY A.