Transcript (02/17/2021)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -
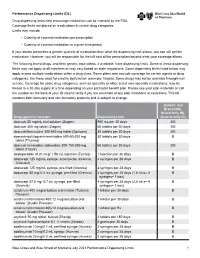
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Rxoutlook® 1St Quarter 2019
® RxOutlook 1st Quarter 2020 optum.com/optumrx a RxOutlook 1st Quarter 2020 Orphan drugs continue to feature prominently in the drug development pipeline In 1983 the Orphan Drug Act was signed into law. Thirty seven years later, what was initially envisioned as a minor category of drugs has become a major part of the drug development pipeline. The Orphan Drug Act was passed by the United States Congress in 1983 in order to spur drug development for rare conditions with high unmet need. The legislation provided financial incentives to manufacturers if they could demonstrate that the target population for their drug consisted of fewer than 200,000 persons in the United States, or that there was no reasonable expectation that commercial sales would be sufficient to recoup the developmental costs associated with the drug. These “Orphan Drug” approvals have become increasingly common over the last two decades. In 2000, two of the 27 (7%) new drugs approved by the FDA had Orphan Designation, whereas in 2019, 20 of the 48 new drugs (42%) approved by the FDA had Orphan Designation. Since the passage of the Orphan Drug Act, 37 years ago, additional regulations and FDA designations have been implemented in an attempt to further expedite drug development for certain serious and life threatening conditions. Drugs with a Fast Track designation can use Phase 2 clinical trials to support FDA approval. Drugs with Breakthrough Therapy designation can use alternative clinical trial designs instead of the traditional randomized, double-blind, placebo-controlled trial. Additionally, drugs may be approved via the Accelerated Approval pathway using surrogate endpoints in clinical trials rather than clinical outcomes. -

Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary
Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary Effective September 1, 2021 Health Plan Products: Kaiser Permanente Bernard J. Tyson School of Medicine, EPO Student Blanket Health Plan offered by Kaiser Permanente Insurance Company For the most current list of covered medications or for help understanding your KPIC insurance plan benefits, including cost sharing for drugs under the prescription drug benefit and under the medical benefit: Call 1-800-533-1833, TTY 711, Monday through Friday, 7 a.m. to 9 p.m. ET Visit kaiserpermanente.org to: • Find a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. • Find an electronic copy of the formulary here. • Get plan coverage information. For cost sharing information for the outpatient prescription drug benefits in your specific plan, please visit kp.org/kpic-websiteTBD The formulary is subject to change and all previous versions of the formulary are no longer in effect. Kaiser Permanente Last updated: September 1, 2021 Table of Contents Informational Section...........................................................................................................................................3 ANTIHISTAMINE DRUGS - Drugs for Allergy.....................................................................................................9 ANTI-INFECTIVE AGENTS - Drugs for Infections........................................................................................... -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

Prescribing Support Update Newsletter
Prescribing Support Update Newsletter VOLUME 11, ISSUE 4 July 2021 IN THIS ISSUE New RDTC website launched New RDTC website 1 launched The RDTC website has been re-developed! The all-new site has the same content you know, but re-organised to make it faster and easier to find what you need with a few Letter to women and girls 1 clicks. The web address hasn’t changed—you can still find us at www.rdtc.nhs.uk. taking sodium valproate The prescribing support section in Spotlight on RDTC 1 particular has been re-arranged so stakeholder reports you can view everything we produce in a therapeutic area with one click, UPDATE : Chloramphenicol 2 eye drops in children under or do a quick search to find exactly 2 years old what you’re looking for. Medicine use in patients 2 For a little more info on using the with swallowing difficulties site, visit our news page. NPSA: Inappropriate antico- 2 You’ll need to re-register to use the agulation prescribing new site, but the form is quick and Webinar discussions on 2 easy to fill in, and we’ll approve your challenging areas of new account as quickly as possible. practice We hope you’ll find the new site New e-learning: 2 easy and intuitive to use, but if you Prescribing for UTIs have any questions or feedback Medicines Availability 3 we’d love to hear it. Get in touch at Information [email protected], or drop us a message on Twitter at What’s new from RDTC 3 @RDTC_Rx New and updated NICE 3 Letter to women and girls taking sodium valproate UK Product launches 3 A letter has been sent to women and girls aged 12-55 who are currently prescribed sodium valproate, and containing important reminders of safety considerations such as contraception, pregnancy and regular prescribing reviews. -

National Direct Drug List
National Direct Drug List Drug list — Five Tier Drug Plan Your prescription benefit comes with a drug list, which is also called a formulary. This list is made up of brand-name and generic prescription drugs approved by the U.S. Food & Drug Administration (FDA). If you are a current Anthem member with questions about your pharmacy benefits, we're here to help. Just call us at the Pharmacy Member Services number on your ID card. Here are a few things to remember: o You can view and search our current drug lists when you visit anthem.com/ca and choose Prescription Benefits. Please note: The formulary is subject to change and all previous versions of the formulary are no longer in effect. o Additional tools and resources are available for current Anthem members to view the most up-to-date list of drugs for your plan - including drugs that have been added, generic drugs and more – by logging in at anthem.com/ca. o Your coverage has limitations and exclusions, which means there are certain rules about what's covered by your plan and what isn't. Already a member? You can view your Certificate/Evidence of Coverage or your Summary Plan Description by logging in at anthem.com/ca and go to My Plan ->Benefits-> Plan Documents. o You and your doctor can use this list as a guide to choose drugs that are best for you. Drugs that aren’t on this list may not be covered by your plan and may cost you more out of pocket. -
FEP 5 Tier Rx Drug Formulary (607) Standard Option
FEP 5 Tier Rx Drug Formulary (607) Standard Option Effective July 1, 2021 The FEP formulary includes the preferred drug list which is comprised of Tier 1, generics and Tier 2, preferred brand-name drugs. Also included in the formulary are Tier 3, non-preferred brand-name drugs, Tier 4, preferred specialty drugs and Tier 5, non-preferred specialty drugs. Ask your physician if there is a generic drug available to treat your condition. If there is no generic drug available, ask your physician to prescribe a preferred brand-name drug. The preferred brand-name drugs within our formulary are listed to identify medicines that are clinically appropriate and cost-effective. Click on the category name in the Table of Contents below to go directly to that page INTRODUCTION ........................................................................................................................................................................................................................ 5 PREFACE ................................................................................................................................................................................................................................... 5 EXCLUDED DRUGS .................................................................................................................................................................................................................. 6 PRIOR APPROVAL .................................................................................................................................................................................................................. -

Enhanced Annual Drug List Dispensing
Enhanced Annual Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral solution (Ziagen) 960 mL per 30 days BG abacavir 300 -

Prescription Drug Formulary
Cleveland Clinic Employee Health Plan Prescription Drug Formulary July 2021 Cleveland Clinic Health Benefit Program Drug Formulary July 2021 Prescription Drug Coverage The listing of a drug in the Formulary does not guarantee coverage if your contract does not cover that category of drugs (e.g., oral contraceptives, infertility agents). Refer to the Benefits and Coverage Clarification section (page13) in the Handbook to determine specific coverage. Approved Medications — Only FDA-approved medications are eligible for coverage. Non-Covered Medications — These drugs are determined by the terms of the member’s group health plan. The following are examples of, but not limited to, drug categories that plans exclude from coverage: drugs used for cosmetic purposes, weight control, promotion of fertility, and sexual dysfunction. Preferred Generic Medications (Non-Specialty; Tier 1) — The Cleveland Clinic Health Benefit Program supports and encourages the use of FDA-approved generic drugs that are both chemically and therapeutically equivalent to manufacturers’ brand name products. Generically equivalent products are safe and effective treatments that offer savings as alternatives to brand name products. This Formulary lists both a generic and a brand name for the purpose of drug recognition. Preferred Brands (Non-Specialty; Tier 2) — An FDA-approved drug of proven therapeutic efficacy and safety and approved by the P&T Committee for inclusion in the Formulary. This Formulary lists both a generic and a brand name for the purpose of drug recognition. Non-Preferred /Non-Formulary Brands and Generics (Tier 3) — Any FDA-approved medication which has been reviewed by the P&T Committee and not added to the Formulary or is new and has not yet been reviewed by the P&T Committee is considered a Non-Preferred/Non-Formulary drug. -

SUMMARY of DRUG LIMITATIONS Medications Listed in This Document May Or May Not Require a Prior Authorization
RON DESANTIS GOVERNOR SIMONE MARSTILLER SECRETARY SUMMARY OF DRUG LIMITATIONS Medications listed in this document may or may not require a prior authorization. Please view the Preferred Drug List at: http://ahca.myflorida.com/Medicaid/Prescribed_Drug/pharm_thera/fmpdl.shtml Summary of Drug Limitations Abilify (aripiprazole) 2mg, 5mg, 20mg, 30mg tablets Minimum age = 6; Maximum of 1 tablet per day Abilify (aripiprazole) 10mg, 15mg tablets Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17 Maximum of 1 tablet per day Abilify (aripiprazole) Discmelt 10mg, 15mg tabs Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17; Maximum of 2 tablets per day Abilify (aripiprazole) 1mg/ml solution Minimum age = 6; Maximum of 15ml per day for ages = 6 - 11; Maximum of 30ml per day for ages = 12-17; Maximum of 30ml per day for ages ≥ 18 Abilify Maintena (aripiprazole) syringe/vial Minimum age = 18; Maximum of 1 syringe or vial every 28 days Abilify Mycite (aripiprazole) Minimum age = 18 Maximum of 30 mg per day Absorica (isotretinoin) capsules/LD capsules Minimum age = 12 Abstral (fentanyl citrate) sublingual tablets Minimum age = 18; Maximum of 4 sublingual tablets per day Acanya (benzoyl peroxide/clindamycin)Gel, gel pump Minimum Age= 12 Accolate (zafirlukast) tablets Maximum of 3 tablets per day Accrufer (ferric maltol) capsule Minimum age = 18 Aciphex (rabeprazole) 5mg, 10mg sprinkle capsules Minimum age = 1; Maximum age = 11; Maximum of 1 capsule per day Aciphex (rabeprazole) 20mg tablets Minimum age = 1; Maximum of 2 tablets per day Updated 06/30/2021 1 RON DESANTIS GOVERNOR SIMONE MARSTILLER SECRETARY SUMMARY OF DRUG LIMITATIONS Medications listed in this document may or may not require a prior authorization.