POS Formulary with Specialty Drug Tier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NG198 Evidence Review E1
1 2 Research recommendations for review question: For people with mild to 3 moderate acne vulgaris what are the most effective treatment options? 4 Research question - physical modalities 5 What is the effectiveness of physical modalities (such as light devices) in the treatment of 6 acne vulgaris or persistent acne vulgaris-related scarring? 7 Why this is important 8 Physical treatments for acne are popular with people because they have the benefit of 9 treating a local area without systemic effects. They can be used in people with co-morbidities 10 or side effects where other treatments are unsuitable. They are currently available in the 11 private sector but there is no standardisation of treatment modalities or duration. Many 12 different physical therapies have been described for acne including: 13 • Comedone extraction 14 • Phototherapy – including UVB, intense pulsed light, blue and red light 15 • Photochemical therapy (e.g. photodynamic therapy) 16 • Laser 17 • Photopneumatic therapy (e.g. intense pulsed light + vacuum) 18 • Photothermal therapy (eg gold nanoparticles +light or laser) 19 Physical treatments are also used for acne scarring. These include: 20 • Punch excision 21 • CO2 laser 22 • Dermabrasion 23 • Radiofrequency (e.g. fractional microneedling, bipolar) 24 Further research is required to determine the most effective physical treatments for acne and 25 acne scarring. This could open the way to wider availability in the NHS. 26 Table 26: Research recommendation rationale Research question What is the effectiveness of physical modalities (such as light devices) in the treatment of acne vulgaris or persistent acne vulgaris-related scarring? Why is this needed Importance to ‘patients’ or the Physical treatments for acne are popular with people because population they have the benefit of treating a local area without systemic effects. -

June 2021 Therapeutic Research Center (TRC) Is the Leading Advisory Service on Drug Therapy and Medication Management
June 2021 Therapeutic Research Center (TRC) is the leading advisory service on drug therapy and medication management. Every month over 400,000 prescribers, pharmacists, and pharmacy technicians rely on our unbiased, evidence-based clinical recommendations to help them improve medication use, prevent medication errors, and improve patient care and outcomes. We also have one of the most extensive CE/CME course offerings in the industry. Our accredited continuing education and continuing medical education courses are trusted and relied on by hundreds of thousands of pharmacists, technicians, and prescribers every month. Therapeutic Research Center does not receive commercial support and does not accept any advertising. It is completely independent and is supported entirely by subscriptions. Credit is reported to CPE Monitor, AAFP, and CE Broker as appropriate. Accreditation Information: Therapeutic Research Center is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing education for physicians. Pharmacist’s Letter / Therapeutic Research Center is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Therapeutic Research Center / Prescriber’s Letter is accredited by the American Association of Nurse Practitioners as an approved provider of nurse practitioner continuing education. Provider number:080517. Select Therapeutic Research Center courses are also acceptable for American Academy of Family Physicians (AAFP) Prescribed credit, American Osteopathic Association (AOA) credit, and American College of Emergency Physicians (ACEP) Category I Credit. Please refer to the detailed accreditation statements available online for each course. Get started at TherapeuticResearchCenter.com. Log in to access your course list or purchase a course or subscription. For additional assistance, please call 209-472-2240 and we’ll be happy to help you. -
3-Local-Anesthetics.Pdf
Overview • Local anesthetics produce a transient and reversible loss of sensation (analgesia) in a circumscribed region of the body without loss of consciousness. • Normally, the process is completely reversible. • Local anesthetics are generally classified as either esters or amides and are usually linked to: – a lipophilic aromatic group – to a hydrophilic, ionizable tertiary (sometimes secondary) amine. • Most are weak bases with pKa ( 8 – 9), and at physiologic pH they are primarily in the charged, cationic form. • The potency of local anesthetics is positively correlated with their lipid solubility, which may vary 16-fold, and negatively correlated with their molecular size. • These anesthetics are selected for use on the basis of: 1. the duration of drug action • Short: 20 min • Intermediate: 1—1.5 hrs • Long: 2—4 hrs 2. effectiveness at the administration site 3. potential for toxicity Mechanism of action Local anesthetics act by blocking sodium channels and the conduction of action potentials along sensory nerves. • Blockade is voltage dependent and time dependent. a. At rest, the voltage-dependent sodium (Na+) channels of sensory nerves are in the resting (closed) state. • Following the action potential the Na+ channel becomes active (open) and then converts to an inactive (closed) state that is insensitive to depolarization. • Following repolarization of the plasma membrane there is a slow reversion of channels from the inactive to the resting state, which can again be activated by depolarization. • During excitation the cationic charged form of local anesthetics interacts preferentially with the inactivated state of the Na+ channels on the inner aspect of the sodium channel to block sodium current and increase the threshold for excitation. -

Comparison of Efficacy of Combination of 2% Ketoconazole
Open Access Original Article Comparison of Topical Treatments in Pityriasis Versicolor Pak Armed Forces Med J 2018; 68 (6): 1725-30 COMPARISON OF EFFICACY OF COMBINATION OF 2% KETOCONAZOLE SOLUTION WASH AND TOPICAL 1% CLOTRIMAZOLE WITH TOPICAL 1% CLOTRIMAZOLE ALONE IN CASES OF PITYRIASIS VERSICOLOR Ayesha Anwar, Naeem Raza, Najia Ahmed, Hyder Ali Awan* Pak Emirates Military Hospital/National University of Medical Sciences (NUMS) Rawalpindi Pakistan, *King Abdul Aziz Naval Base, Jubail, Saudia Arabia ABSTRACT Objective: Comparison of efficacy of combination comprising 2% ketoconazole solution wash plus topical 1% clotrimazole versus topical 1% clotrimazole alone in management of patients with Pityriasis versicolor. Study Design: Randomized controlled trial. Place and Duration of Study: Dermatology department, Pak Emirates Military Hospital Rawalpindi, from Oct 2016 to Apr 2017. Material and Methods: Sixty patients of Pityriasis versicolor, both male and female were included in study. Diagnosis of Pityriasis versicolor was made clinically and confirmed microscopically by examining skin scrapings for fungal hyphae. Patients with concomitant systemic illnesses or those who had received anti-fungal in last three months were excluded from study. Random number tables were used to allocate patients to the two treatment groups. Group A received 2% ketoconazole shampoo twice per week for four weeks plus topical 1% clotrimazole twice daily application for 2 weeks. Group B received only topical therapy with 1% clotrimazole cream applied twice daily for 2 weeks. Assessment of treatment efficacy was done by clinical examination of patient and microscopy of skin scrapping for fungal hyphaedone at baseline and at end of study (4 weeks of treatment). A negative clinical examination and negative skin scrapping for fungal hyphae was considered effective therapeutic response. -

Nootropic Concepts
Nootropic concepts intelligent ingredient for gut health Nootropics definition Nootropic is a substance that enhances cognition and memory and facilitates learning. Nootropics work in many ways to produce a range of benefits across memory, focus, attention, motivation, relaxation, mood, alertness, stress resistance. Bluenesse® Bluenesse® – an innovative, exclusive lemon balm extract, Melissa officinalis (L.) – supports your mental health. It has an immediate effect on focus, concentration, and memory. Furthermore, it supports a calm and good mood and helps to balance the stress hormone cortisol. Bluenesse® - Nootropics concepts Nootropic benefits of Bluenesse® and the underlying mode of action: Bluenesse® - Nootropics concepts – detailed information: Several, double blind, randomized, placebo-controlled, crossover human study results have shown that Bluenesse® supports nootropic effects on demand. Below, the investigated parameter and mode of action is explained per each nootropic application. Vital Solutions GmbH, Hausingerstrasse 6, D-40764 Langenfeld, +49 (0) 2173 109 82 02 [email protected] www.vitalsolutions.biz . Nootropic concepts Cognitive performance support and enhancing learning & memory: Bluenesse® significantly improves cognitive performance, particularly alertness, working memory and mathematic processing by improving the efficiency of neuronal communication. It activates Muscarinic receptors, which are responsible for the efficient flow of information between neurological cells, so-called “oscillation”, leading to -

Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -

Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy
Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy October 2014 This document is coded JBDS 08 in the series of JBDS documents Other JBDS documents: Admissions avoidance and diabetes: guidance for clinical commissioning groups and clinical team; December 2013, JBDS 07 The management of the hyperosmolar hyperglycaemic state (HHS) in adults with diabetes; August 2012, JBDS 06 Glycaemic management during the inpatient enteral feeding of stroke patients with diabetes; June 2012, JBDS 05 Self-management of diabetes in hospital; March 2012, JBDS 04 Management of adults with diabetes undergoing surgery and elective procedures: improving standards; April 2011, JBDS 03 The Management of Diabetic Ketoacidosis in Adults; revised September 2013, JBDS 02 The hospital management of hypoglycaemia in adults with diabetes mellitus; revised September 2013, JBDS 01 These documents are available to download from: ABCD website: www.diabetologists-abcd.org.uk/JBDS/JBDS.htm Diabetes UK website: www.diabetes.org.uk Contents Page Foreword 4 Authorship and acknowledgments 5-6 Introduction 7 Steroids - mechanism of action 8 Steroid therapy – impact on blood glucose 9 Glucose targets 10 Glucose monitoring 11 Diabetes treatment options 12-13 Treatment of steroid induced hyperglycaemia 14-15 Hospital discharge 16-17 Steroid treatment in pregnancy 18 Steroid treatment in end of life 19 Audit standards 20 Controversial areas 21 References 22 Appendix 1 – Algorithm to show treatment of steroid 23 induced diabetes Appendix 2 – Algorithm to show management of patients 24 with diabetes on once daily steroids Appendix 3 – End of life steroid management 25 Appendix 4 – Patient letter – Glucose monitoring and 26 steroid use 3 Foreword This is the latest in the series of Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guidelines, and focuses on steroid induced hyperglycaemia and steroid induced diabetes. -
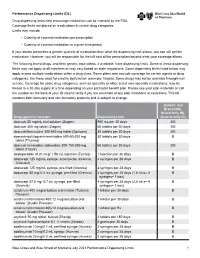
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Soliqua – Criteria
Criteria Based Consultation Prescribing Program CRITERIA FOR DRUG COVERAGE Insulin glargine; lixisenatide (Soliqua) subcutaneous injection Initiation (new start) criteria: Non-formulary lixisenatide/glargine (Soliqua) will be covered on the prescription drug benefit when the following criteria are met: Must meet criteria for both individual agents lixisenatide and insulin glargine. Criteria for lixisenatide: • Diagnosis of Type 2 Diabetes Mellitus • Intolerance to preferred GLP-1 agonists liraglutide (Victoza) AND injectable semaglutide (Ozempic) • No personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) • No personal history of gastroparesis • On maximally tolerated metformin dose (dose appropriate per renal function) or allergy or intolerance* to metformin (includes both metformin IR and XR) • And meets one of the following criteria: o Inadequate glycemic response on both basal and bolus insulin despite high dose requirements (total daily insulin dose of 1.5 units per kilogram of body weight or more OR greater than 200 units) OR o Has experienced recurrent nocturnal hypoglycemia with basal insulin defined as: 3 or more episodes of nocturnal hypoglycemia (BG less than 70 mg/dL) over the preceding 30 days that persists despite basal insulin dose reduction (including decrease in NPH dose and subsequent switch to and dose adjustment of insulin glargine) Criteria for insulin glargine • Use in patients with type 1 diabetes mellitus as basal insulin -OR- • Use in patients with -

Nootropics for Healthy Individuals
Trinity College Trinity College Digital Repository Trinity Publications (Newspapers, Yearbooks, The First-Year Papers (2010 - present) Catalogs, etc.) 2015 Nootropics for Healthy Individuals Jin Pyo Jeon Trinity College, Hartford Connecticut, [email protected] Follow this and additional works at: https://digitalrepository.trincoll.edu/fypapers Part of the Chemicals and Drugs Commons Recommended Citation Jeon, Jin Pyo, "Nootropics for Healthy Individuals". The First-Year Papers (2010 - present) (2015). Trinity College Digital Repository, Hartford, CT. https://digitalrepository.trincoll.edu/fypapers/61 Nootropics for Healthy Individuals Jin Pyo Jeon With recent advances in fields like biotechnology and genetic engineering, the concern for a just and equal distribution of human enhancement technologies undoubtedly became one of the most significant ethical dilemmas of the 21st century. And with the sudden rise of cognitive enhancement drug (otherwise called as nootropics) use in society, the need for developing policies to address these dilemmas have now become an urgent issue that the scientific and political community must confront. Once only used by the few with special needs or neurological disorders, nootropics are now being utilized by a significant part of the population for cognitive enhancement, inciting a debate of the regulation and legalization of nootropics. Nonetheless, given the currently known benefits and risks of nootropics, the mechanisms through which nootropics function, and the ineffectiveness of policy restrictions, it would be more pragmatic to inform and allow for the non-prescription uses for some nootropics rather than to restrict its use. Benefits, Risks, and Viability of the Use of Nootropics Among many other nootropics, two drugs have become the de facto nootropics for many healthy individuals: modafinil (commercially known as Provigil) and methylphenidate (commercially known as Ritalin). -

OREXIN ANTAGONISTS BELSOMRA (Suvorexant), DAYVIGO (Lemborexant)
OREXIN ANTAGONISTS BELSOMRA (suvorexant), DAYVIGO (lemborexant) RATIONALE FOR INCLUSION IN PA PROGRAM Background Belsomra (suvorexant) and Dayvigo (lemborexant) are orexin receptor antagonists used to treat difficulty in falling and staying asleep (insomnia). Orexins are chemicals that are involved in regulating the sleep-wake cycle and play a role in keeping people awake (1-2). Regulatory Status FDA-approved indication: Orexin receptor antagonists are indicated for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance (1-2). Orexin Antagonists are contraindicated in patients with narcolepsy (1-2). Orexin Antagonists are central nervous system (CNS) depressants that can impair daytime wakefulness even when used as prescribed. Medications that treat insomnia can cause next-day drowsiness and impair driving and other activities that require alertness. Orexin Antagonists can impair driving skills and may increase the risk of falling asleep while driving. People can be impaired even when they feel fully awake. Patients should also be made aware of the potential for next-day driving impairment, because there is individual variation in sensitivity to the drug (1-2). The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or mental illness that should be evaluated (1-2). Warnings and precautions that should be discussed with the patient on Orexin Antagonist therapy include adverse reactions on abnormal thinking and behavioral changes (such as amnesia, anxiety, hallucinations and other neuropsychiatric symptoms), complex behaviors (such as sleep- driving, preparing and eating food, or making phone calls), dose-dependent increase in suicidal ideation, and sleep paralysis which is the inability to move or speak for up to several minutes during sleep-wake transitions (1-2).