January 2021 Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention and Management of Intravesical BCG-Related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate In
Prevention and Management of Intravesical BCG-related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate in Patients with Non-Muscle-Invasive Bladder Cancer: A Randomized Controlled Trial Samer Shamout, Simon Tanguay, Maurice Anidjar, Lysanne Campeau CQI Research Grant 2017 INTRODUCTION METHODS / INTERVENTIONS PATIENT IMPACT Study Design • Bacillus Calmette-Guerin (BCG) remains the most effective • Phase 2, double-blind, randomized, placebo-controlled, parallel-group • Improve patient experience by identifying predictor markers prophylactic treatment of intermediate and high-risk non- study rendering patients at higher risk of developing BCG-related muscle invasive bladder cancer. • Multicentre trial involving two partner sites: (Jewish General Hospital, LUTS. McGill University Health Centre) • 30 to 60% of bladder cancer patients experienced systemic • This will guide patient counselling before treatment to inform Outcomes them about associated risks and benefits of the BCG and local adverse events of variable severity following Primary outcomes (change from baseline to end of treatment): treatment. intravesical BCG therapy • Mean change in number of urgency episodes/24hr (3-day bladder diary) • Only 16 to 29% of patients completed all the three-year BCG • Mean change in ICIQ-LUTSqol score • We will determine the role of prophylactic PPS to prevent these treatment regimen. • Mean change in OAB-V8 score LUTS from occurring, particularly if patients are considered at Secondary outcomes (change from baseline to end of treatment): higher risk, and therefore improve patient experience. • Guidelines recommend medications, such as oxybutynin • Mean change in VAS score only phenazopyridine, and propantheline bromide for short term • Mean change in urinary inflammatory markers (TRAIL, IFN, IL-2, IL-10) • The improvement measurements are validated in the symptomatic relief. -

Abstracts from the 9Th Biennial Scientific Meeting of The
International Journal of Pediatric Endocrinology 2017, 2017(Suppl 1):15 DOI 10.1186/s13633-017-0054-x MEETING ABSTRACTS Open Access Abstracts from the 9th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES) and the 50th Annual Meeting of the Japanese Society for Pediatric Endocrinology (JSPE) Tokyo, Japan. 17-20 November 2016 Published: 28 Dec 2017 PS1 Heritable forms of primary bone fragility in children typically lead to Fat fate and disease - from science to global policy a clinical diagnosis of either osteogenesis imperfecta (OI) or juvenile Peter Gluckman osteoporosis (JO). OI is usually caused by dominant mutations affect- Office of Chief Science Advsor to the Prime Minister ing one of the two genes that code for two collagen type I, but a re- International Journal of Pediatric Endocrinology 2017, 2017(Suppl 1):PS1 cessive form of OI is present in 5-10% of individuals with a clinical diagnosis of OI. Most of the involved genes code for proteins that Attempts to deal with the obesity epidemic based solely on adult be- play a role in the processing of collagen type I protein (BMP1, havioural change have been rather disappointing. Indeed the evidence CREB3L1, CRTAP, LEPRE1, P4HB, PPIB, FKBP10, PLOD2, SERPINF1, that biological, developmental and contextual factors are operating SERPINH1, SEC24D, SPARC, from the earliest stages in development and indeed across generations TMEM38B), or interfere with osteoblast function (SP7, WNT1). Specific is compelling. The marked individual differences in the sensitivity to the phenotypes are caused by mutations in SERPINF1 (recessive OI type obesogenic environment need to be understood at both the individual VI), P4HB (Cole-Carpenter syndrome) and SEC24D (‘Cole-Carpenter and population level. -

Impact of Tamsulosin, Tolterodine and Drug-Combination On
African Journal of Urology (2017) 23, 28–32 African Journal of Urology Official journal of the Pan African Urological Surgeon’s Association web page of the journal www.ees.elsevier.com/afju www.sciencedirect.com Original article Impact of Tamsulosin, Tolterodine and drug-combination on the outcomes of lower urinary tract symptoms secondary to post-ureteroscopy ureteral stent: A prospective randomized controlled clinical study a b,∗ a a O. Abdelkader , K. Mohyelden , M.H. Sherif , A.H. Metwaly , b c d a H. Aldaqadossi , A. Shelbaya , H. Khairy , A. Elnashar a Department of Urology, Suez Canal University, Ismailia, Egypt b Department of Urology, Fayoum University, Fayoum, Egypt c Student Hospital, Cairo University, Cairo, Egypt d Department of Urology, Cairo University, Cairo, Egypt Received 17 March 2016; received in revised form 23 June 2016; accepted 23 June 2016 Available online 18 November 2016 KEYWORDS Abstract Anticholinergics; Objectives: To compare the role of alpha-blocker (Tamsulosin) monotherapy, anticholinergic (Tolterodine) ␣-Adrenergic blockers; monotherapy or combination of both drugs versus analgesics in improving post-ureteroscopy (URS) lower Ureter stent; urinary tract symptoms related to double-J ureteral stent. Ureteroscopy Patients and methods: Between January 2009 and June 2013, 160 consecutive patients with ureteric stones were included in this study at 2 tertiary care centers’. Patients were randomized into 4 groups; group A (n = 40) received 0.4 mg Tamsulosin once a day, group B (n = 40) received 4 mg Tolterodine once a day, group C (n = 40) received Tamsulosin 0.4 mg and Tolterodine 4 mg once a day and group D (n = 40) as a control group, received placebo once a day. -

Phenazopyridine | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Phenazopyridine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US AZO Urinary Pain Relief [OTC]; Baridium [OTC] [DSC]; Pyridium; Urinary Pain Relief [OTC] Brand Names: Canada Phenazo; Pyridium What is this drug used for? It is used to ease pain from a bladder infection. It is used to treat signs of urinary problems. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Kidney disease or liver disease. This is not a list of all drugs or health problems that interact with this drug. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems. Phenazopyridine 1/5 Do not start, stop, or change the dose of any drug without checking with your doctor. What are some things I need to know or do while I take this drug? Tell all of your health care providers that you take this drug. This includes your doctors, nurses, pharmacists, and dentists. This drug is not to be used instead of an antibiotic. -
Study Protocol
CLINICAL STUDY PROTOCOL Study Title: An International Phase 3, Randomized, Double-Blind, Placebo- and Active (Tolterodine)-Controlled Multicenter Study to Evaluate the Safety and Efficacy of Vibegron in Patients with Symptoms of Overactive Bladder Protocol Number: RVT-901-3003 Compound Name and/or Vibegron Number: Indication Treatment of Overactive Bladder Sponsor: Urovant Sciences GmbH Viaduktstrasse 8 4051 Basel Switzerland Development Phase: 3 Regulatory Identifier(s): IND# 106,410 EudraCT# 2017-003293-14 Current Version and Version 3.0; 15 NOV 2018 Effective Date: Previous Version(s) and Version 2.1; 12 FEB 2018 Effective Date(s): Version 2.0; 30 JAN 2018 Version 1.2; 01 NOV 2017 Version 1.1; 05 OCT 2017 Version 1.0; 29 SEP 2017 Study Director: , , Clinical Development Confidentiality Statement The information contained in this document, particularly unpublished data, is the property or under control of Urovant Sciences GmbH, and is provided to you in confidence as an investigator, potential investigator or consultant, for review by you, your staff, and an applicable Institutional Review Board or Independent Ethics Committee. The information is only to be used by you in connection with authorized clinical studies of the investigational drug described in the protocol. You will not disclose any of the information to others without written authorization from Urovant Sciences GmbH. except to the extent necessary to obtain informed consent from those persons to whom the drug may be administered. Confidential Page | 1 NCT Number: NCT03492281 This NCT number has been applied to the document for purposes of posting on clinicaltrials.gov Clinical Study Protocol RVT-901-3003 Urovant Sciences GmbH Effective: 15 NOV 2018 SUMMARY OF CHANGES Version Location Description of Change 3.0 Global Minor typographical/formatting errors were corrected. -
Performance Drug List Dispensing Limits
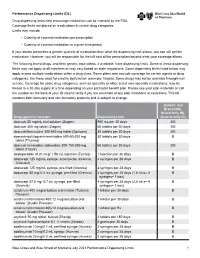
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Psychiatric History and Overactive Bladder Symptom Severity in Ambulatory Urogynecological Patients
Journal of Clinical Medicine Article Psychiatric History and Overactive Bladder Symptom Severity in Ambulatory Urogynecological Patients Artur Rogowski 1,2,* , Maria Krowicka-Wasyl 2, Ewa Chotkowska 2, Tomasz Kluz 3, Andrzej Wróbel 4 , Dominika Berent 5 , Paweł Mierzejewski 6, Halina Sienkiewicz-Jarosz 7, Adam Wichniak 8, Marcin Wojnar 9 , Jerzy Samochowiec 10 , Katarzyna Kilis-Pstrusinska 11 and Przemyslaw Bienkowski 9 1 Faculty of Medicine, Collegium Medicum, Cardinal Stefan Wyszynski University in Warsaw, 01-938 Warsaw, Poland 2 Department of Obstetrics and Gynecology, Mother and Child Institute, 01-211 Warsaw, Poland; [email protected] (M.K.-W.); [email protected] (E.C.) 3 Department of Gynecology and Obstetrics, Institute of Medical Sciences, Medical College of Rzeszow University, 35-310 Rzeszów, Poland; [email protected] 4 Second Department of Gynecology, Medical University of Lublin, Jaczewskiego 8, 20-954 Lublin, Poland; [email protected] 5 Regional Psychiatric Hospital Drewnica, 05-091 Zabki, Poland; [email protected] 6 Departments of Pharmacology, Institute of Psychiatry and Neurology, 02-957 Warsaw, Poland; [email protected] 7 Department of Neurology I, Institute of Psychiatry and Neurology, 02-957 Warsaw, Poland; [email protected] 8 Department of Psychiatry III, Institute of Psychiatry and Neurology, 02-957 Warsaw, Poland; Citation: Rogowski, A.; [email protected] 9 Krowicka-Wasyl, M.; Chotkowska, E.; Department of Psychiatry, Medical University of Warsaw, 02-091 Warsaw, Poland; Kluz, T.; Wróbel, A.; Berent, D.; [email protected] (M.W.); [email protected] (P.B.) 10 Mierzejewski, P.; Sienkiewicz-Jarosz, Department of Psychiatry, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] 11 Department of Pediatric Nephrology, Wroclaw Medical University, 02-091 Wroclaw, Poland; H.; Wichniak, A.; Wojnar, M.; et al. -

62. Deutscher Kongress Für Endokrinologie
62. Deutscher Kongress für Endokrinologie dge2019.de TAGUNGS- FÜHRER 20.–22. März 2019 © Conny Blaack M.A., kunst & mediendesign, 37130 Gleichen/Bremke GÖTTINGEN Kongresspräsidentin: HAUPTTHEMEN Prof. Dr. Heide Siggelkow Knochen als hormonelles Organ Wissenschaftliche Kongresssekretärin: Vitamin D, das endokrine und Dr. Katja Gollisch metabolische System Kongressorganisation: Individualisierte Osteoporosetherapie EndoScience Endokrinologie Service GmbH Update Andrologie nach Berthold Endokrine Onkologie Mittwoch, 20.03.2019 Programmübersicht Raum Lichtenberg Raum Gauss Raum Heisenberg Raum Gebrüder Grimm Raum Göttinger Sieben 09:00 – 10:30 Vorstandssitzung 09:00 – 12:00 S. 18 Sektionstreffen YARE / MuSkITYRS 10:30 – 11:30 Pressekonferenz S. 18 S. 18 11:30 – 12:30 Eröffung der Industrieausstellung S. 19 12:30 – 13:00 Kongresseröffnung S. 19 13:00 – 13:45 Aktuelles zur Mikrobiom-Hor- mon-Achse: Prof. Dr. Bar- bara Obermayer- Pietsch, Graz, Österreich S. 19 13:45 – 14:15 Lunch 13:45 – 14:45 Akromegalieregister 14:00 – 15:00 Neugründung 14:15 – 15:15 14:00 – 16:00 S. 19 der AG Labor Satelliten Symposium 1: 14:15 – 15:45 S1: Vom Tiermodell zum UCB Pharma GmbH S. 20 Satelliten Symposium 2: Menschen – Aktuelles S. 22 Merck Serono GmbH zur Reproduktions- endokrinologie S. 22 15:45 – 16:15 Kaffeepause S. 20 16:00 – 17:30 16:15 – 17:15 16:00 – 17:30 S2: Hypophysen- Sektionstreffen MTE 1 16:15 – 17:45 15:00 – 19:15 erkrankungen Reproduktions- MTE 2 S3 Hauptthema: Endokrinologie in der Schwangerschaft biologie und -medizin Cortisol und Knochen: für die Praxis S. 24 Freunde oder Feinde? update 2019 S. 23 S. 23/24 (kostenpflichtig) separate Anmeldung S. -

Gemtesa® (Vibegron) – New Drug Approval
Gemtesa® (vibegron) – New drug approval • On December 23, 2020, Urovant Sciences announced the FDA approval of Gemtesa (vibegron), for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults. • OAB is a common condition that occurs when the bladder muscle contracts involuntarily. Over 30 million Americans are estimated to suffer from bothersome symptoms of OAB. • Gemtesa is a selective human beta-3 adrenergic receptor agonist. Activation of the beta-3 adrenergic receptor increases bladder capacity by relaxing the detrusor smooth muscle during bladder filling. • The efficacy of Gemtesa was established in a 12-week, double-blind, randomized, placebo- controlled, and active-controlled study in 1,515 patients with OAB (urge urinary incontinence, urgency, and urinary frequency). The co-primary endpoints were change from baseline in average daily number of micturitions and average daily number of urge urinary incontinence (UUI) episodes at week 12. — The change from baseline at week 12 in the average daily number of micturitions was -1.8 and -1.3 for Gemtesa and placebo, respectively (difference of -0.5, 95% CI: -0.8, -0.2; p < 0.001). — The change from baseline at week 12 in the average daily number of UUI episodes was -2.0 and -1.4 for Gemtesa and placebo, respectively (difference of -0.6, 95% CI: -0.9, -0.3; p < 0.0001). • A warning and precaution for Gemtesa is urinary retention. • The most common adverse reactions (2%) with Gemtesa use were headache, urinary tract infection, nasopharyngitis, diarrhea, nausea, and upper respiratory tract infection. • The recommended dose of Gemtesa is one 75 mg tablet orally, once daily with or without food. -

Drug Formulary
University of Arkansas March 2017 Use of generic drugs can save both you and your health plan money. This list is not all-inclusive and is not a guarantee of coverage. Plan Benefit design is the final determinate of coverage. Certain drugs (*) may be subject to Prior Authorization (PA), Quantity Limits (QL), Step Therapy (ST), or Reference Based Pricing (RBP) requirements according to Benefit Design. Unless noted, multisource brand drugs (brand drugs with generic equivalent) are covered at 100% copay. If you have any questions about these requirements or other formulary questions, please contact a MedImpact Healthcare customer service representative at 800-788-2949. This list represents brand products in CAPS, branded generics in upper- and lowercase Italics, and generic products in lowercase italics. Drug Type Tier 1 Tier 2 Tier 3 Anti-Infectives Antibiotics – Cephalosporins cefaclor, cefadroxil, cefdinir, CEFTIN susp, SUPRAX 400mg only* (Quantity Limit) cefpodoxime, cefprozil, (QL) cefditoren,cefuroxime, Note: all other Suprax strengths are 100% cephalexin copay Antibiotics - Macrolides azithromycin, clarithromycin, ERY-TAB, ZMAX susp clarithromycin ext-rel, PCE erythromycin delayed-rel, erythromycin ethylsuccinate, erythromycin stearate Antibiotics - Fluoroquinolones ciprofloxacin, ciprofloxacin ext- FACTIVE rel, levofloxacin ,moxifloxacin Antibiotics - Penicillins amoxicillin, amoxicillin- clavulanate, dicloxacillin, penicillin VK Antibiotics – Other* (Prior clindamycin HCl, doxycycline ZYVOX susp*(PA) Authorization) hyclate, linezolid* -

Rxoutlook® 1St Quarter 2019
® RxOutlook 1st Quarter 2020 optum.com/optumrx a RxOutlook 1st Quarter 2020 Orphan drugs continue to feature prominently in the drug development pipeline In 1983 the Orphan Drug Act was signed into law. Thirty seven years later, what was initially envisioned as a minor category of drugs has become a major part of the drug development pipeline. The Orphan Drug Act was passed by the United States Congress in 1983 in order to spur drug development for rare conditions with high unmet need. The legislation provided financial incentives to manufacturers if they could demonstrate that the target population for their drug consisted of fewer than 200,000 persons in the United States, or that there was no reasonable expectation that commercial sales would be sufficient to recoup the developmental costs associated with the drug. These “Orphan Drug” approvals have become increasingly common over the last two decades. In 2000, two of the 27 (7%) new drugs approved by the FDA had Orphan Designation, whereas in 2019, 20 of the 48 new drugs (42%) approved by the FDA had Orphan Designation. Since the passage of the Orphan Drug Act, 37 years ago, additional regulations and FDA designations have been implemented in an attempt to further expedite drug development for certain serious and life threatening conditions. Drugs with a Fast Track designation can use Phase 2 clinical trials to support FDA approval. Drugs with Breakthrough Therapy designation can use alternative clinical trial designs instead of the traditional randomized, double-blind, placebo-controlled trial. Additionally, drugs may be approved via the Accelerated Approval pathway using surrogate endpoints in clinical trials rather than clinical outcomes.