Pharmacotherapy for Interstitial Cystitis/Bladder Pain Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention and Management of Intravesical BCG-Related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate In
Prevention and Management of Intravesical BCG-related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate in Patients with Non-Muscle-Invasive Bladder Cancer: A Randomized Controlled Trial Samer Shamout, Simon Tanguay, Maurice Anidjar, Lysanne Campeau CQI Research Grant 2017 INTRODUCTION METHODS / INTERVENTIONS PATIENT IMPACT Study Design • Bacillus Calmette-Guerin (BCG) remains the most effective • Phase 2, double-blind, randomized, placebo-controlled, parallel-group • Improve patient experience by identifying predictor markers prophylactic treatment of intermediate and high-risk non- study rendering patients at higher risk of developing BCG-related muscle invasive bladder cancer. • Multicentre trial involving two partner sites: (Jewish General Hospital, LUTS. McGill University Health Centre) • 30 to 60% of bladder cancer patients experienced systemic • This will guide patient counselling before treatment to inform Outcomes them about associated risks and benefits of the BCG and local adverse events of variable severity following Primary outcomes (change from baseline to end of treatment): treatment. intravesical BCG therapy • Mean change in number of urgency episodes/24hr (3-day bladder diary) • Only 16 to 29% of patients completed all the three-year BCG • Mean change in ICIQ-LUTSqol score • We will determine the role of prophylactic PPS to prevent these treatment regimen. • Mean change in OAB-V8 score LUTS from occurring, particularly if patients are considered at Secondary outcomes (change from baseline to end of treatment): higher risk, and therefore improve patient experience. • Guidelines recommend medications, such as oxybutynin • Mean change in VAS score only phenazopyridine, and propantheline bromide for short term • Mean change in urinary inflammatory markers (TRAIL, IFN, IL-2, IL-10) • The improvement measurements are validated in the symptomatic relief. -

Impact of Tamsulosin, Tolterodine and Drug-Combination On
African Journal of Urology (2017) 23, 28–32 African Journal of Urology Official journal of the Pan African Urological Surgeon’s Association web page of the journal www.ees.elsevier.com/afju www.sciencedirect.com Original article Impact of Tamsulosin, Tolterodine and drug-combination on the outcomes of lower urinary tract symptoms secondary to post-ureteroscopy ureteral stent: A prospective randomized controlled clinical study a b,∗ a a O. Abdelkader , K. Mohyelden , M.H. Sherif , A.H. Metwaly , b c d a H. Aldaqadossi , A. Shelbaya , H. Khairy , A. Elnashar a Department of Urology, Suez Canal University, Ismailia, Egypt b Department of Urology, Fayoum University, Fayoum, Egypt c Student Hospital, Cairo University, Cairo, Egypt d Department of Urology, Cairo University, Cairo, Egypt Received 17 March 2016; received in revised form 23 June 2016; accepted 23 June 2016 Available online 18 November 2016 KEYWORDS Abstract Anticholinergics; Objectives: To compare the role of alpha-blocker (Tamsulosin) monotherapy, anticholinergic (Tolterodine) ␣-Adrenergic blockers; monotherapy or combination of both drugs versus analgesics in improving post-ureteroscopy (URS) lower Ureter stent; urinary tract symptoms related to double-J ureteral stent. Ureteroscopy Patients and methods: Between January 2009 and June 2013, 160 consecutive patients with ureteric stones were included in this study at 2 tertiary care centers’. Patients were randomized into 4 groups; group A (n = 40) received 0.4 mg Tamsulosin once a day, group B (n = 40) received 4 mg Tolterodine once a day, group C (n = 40) received Tamsulosin 0.4 mg and Tolterodine 4 mg once a day and group D (n = 40) as a control group, received placebo once a day. -

Phenazopyridine | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Phenazopyridine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US AZO Urinary Pain Relief [OTC]; Baridium [OTC] [DSC]; Pyridium; Urinary Pain Relief [OTC] Brand Names: Canada Phenazo; Pyridium What is this drug used for? It is used to ease pain from a bladder infection. It is used to treat signs of urinary problems. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Kidney disease or liver disease. This is not a list of all drugs or health problems that interact with this drug. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems. Phenazopyridine 1/5 Do not start, stop, or change the dose of any drug without checking with your doctor. What are some things I need to know or do while I take this drug? Tell all of your health care providers that you take this drug. This includes your doctors, nurses, pharmacists, and dentists. This drug is not to be used instead of an antibiotic. -

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -

Intravesical Cocktails PBS/IC
International Painful Bladder Foundation Interstitial Cystitis/Painful Bladder Syndrome Anaesthetic intravesical cocktails 1. Anaesthetic cocktail – Robert Moldwin, MD 1:1 mixture of 0.5% Marcaine and 2% Lidocaine jelly – about 40 cc total. To this solution are added: Heparin sulphate 10,000 IU Triamcinolone 40 mg Gentamycin 80 mg or a post-procedural prophylactic antibiotic. Administration: Patients are instructed to hold the solution for about 30 minutes, then to void. When given as a diagnostic test, patients will generally sense relief of pain within 5-10 minutes. The only (rare) problems that we’ve encountered are the following: Patients may experience “rebound” pain once the solution has worn off (within 3-5 hours). This generally resolves with continued instillations. When given as therapy, we usually administer the cocktail on a weekly basis for 8-12 weeks. This is the length of time usually needed to get a prolonged response. Then, the duration between instillations is increased to q 2 weeks to q 3 weeks, etc., ultimately with the goal of discontinuance. Patients may experience urinary retention requiring catheterization. This seems to be particularly a problem in patients who appear to have pre-existing voiding dysfunction, those patients who initially present with a poor urinary flow rate, an interrupted urinary stream, etc. The urinary retention can usually be circumvented by delivering a lower total volume. 2. Marcaine with steroid cocktail – Nagendra Mishra, MD Marcaine 40 ml Heparin sulphate 10,000 IU Dexamethasone 2 cc Sodium bicarbonate 20 ml Administration: This cocktail should be held in the bladder for 20 minutes. It should be administered every 15 days for a total of 6 treatments and then as needed. -

Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: a Randomized
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6367210 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized... Article in Veterinary Surgery · May 2007 DOI: 10.1111/j.1532-950X.2007.00256.x · Source: PubMed CITATIONS READS 16 103 4 authors, including: Steven C Budsberg Mary Sarah Bergh University of Georgia Iowa State University 129 PUBLICATIONS 3,106 CITATIONS 22 PUBLICATIONS 366 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Steven C Budsberg on 11 March 2014. The user has requested enhancement of the downloaded file. Veterinary Surgery 36:234–244, 2007 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized, Placebo-Controlled Clinical Trial STEVEN C. BUDSBERG, DVM, MS, Diplomate ACVS, MARY SARAH BERGH, DVM, LISA R. REYNOLDS, BS, and HEATHER K. STREPPA, DVM, Diplomate ACVS Objective—To evaluate the efficacy of pentosan polysulfate (PPS) for improving the recovery period and mitigate the progression of osteoarthritis (OA) of the canine stifle after extracapsular stabilization of cranial cruciate ligament (CCL) injuries. Study Design—Randomized, blinded, placebo-controlled clinical trial. Animals—Dogs (n ¼ 40) with unilateral CCL instability. Methods—Each dog had an extracapsular stabilization of the stifle with or without partial men- iscectomy. Dogs were divided into 4 groups based on preoperative radiographic assessment and whether a partial meniscectomy was performed. Dogs were randomly assigned to either (3 mg/kg) PPS or placebo treatment in each group, and then injected subcutaneously weekly for 4 weeks. -
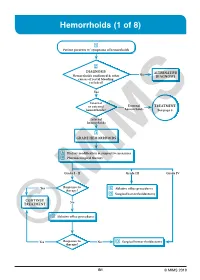
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

Management of Joint Disease in the Sport Horse
1 MANAGEMENT OF JOINT DISEASE IN THE SPORT HORSE Management of Joint Disease in the Sport Horse C. WAYNE MCILWRAITH Colorado State University, Ft. Collins, Colorado INTRODUCTION The joint is an organ, and there are a number of ways in which traumatic damage occurs, ultimately resulting in degradation of articular cartilage. It was recognized in 1966 that articular cartilage change that accompanied osteochondral fragmentation could also be associated with concurrent traumatic damage to the attachment of the joint capsule and ligaments (Raker et al., 1966). However, there was little association made between primary disease in the synovial membrane and fibrous joint capsule and the development of osteoarthritic change in the articular cartilage until an experimental study demonstrated that cartilage degradation could occur in the horse in the absence of instability or trau- matic disruption of tissue and that loss of glycosaminoglycan (GAG) staining was associated with early morphologic breakdown at the surface of the cartilage (McIlwraith and Van Sickle, 1984). Surveys have confirmed that approximately 60% of lameness problems are related to osteoarthritis (National Animal Health Monitoring Systems, 2000; Caron and Genovese, 2003). Rapid resolution of synovitis and capsuli- tis is a critical part of the medical treatment of joint disease because of the principal role of synovitis in causing cartilage matrix breakdown. The goal of treatment of traumatic entities of the joint is twofold: (1) returning the joint to normal as quickly as possible, and (2) preventing the occurrence or reduction of the severity of osteoarthritis. In other words, treatment is intended to (1) reduce pain (lameness), and (2) minimize progression of joint deterioration. -

Drug Formulary
University of Arkansas March 2017 Use of generic drugs can save both you and your health plan money. This list is not all-inclusive and is not a guarantee of coverage. Plan Benefit design is the final determinate of coverage. Certain drugs (*) may be subject to Prior Authorization (PA), Quantity Limits (QL), Step Therapy (ST), or Reference Based Pricing (RBP) requirements according to Benefit Design. Unless noted, multisource brand drugs (brand drugs with generic equivalent) are covered at 100% copay. If you have any questions about these requirements or other formulary questions, please contact a MedImpact Healthcare customer service representative at 800-788-2949. This list represents brand products in CAPS, branded generics in upper- and lowercase Italics, and generic products in lowercase italics. Drug Type Tier 1 Tier 2 Tier 3 Anti-Infectives Antibiotics – Cephalosporins cefaclor, cefadroxil, cefdinir, CEFTIN susp, SUPRAX 400mg only* (Quantity Limit) cefpodoxime, cefprozil, (QL) cefditoren,cefuroxime, Note: all other Suprax strengths are 100% cephalexin copay Antibiotics - Macrolides azithromycin, clarithromycin, ERY-TAB, ZMAX susp clarithromycin ext-rel, PCE erythromycin delayed-rel, erythromycin ethylsuccinate, erythromycin stearate Antibiotics - Fluoroquinolones ciprofloxacin, ciprofloxacin ext- FACTIVE rel, levofloxacin ,moxifloxacin Antibiotics - Penicillins amoxicillin, amoxicillin- clavulanate, dicloxacillin, penicillin VK Antibiotics – Other* (Prior clindamycin HCl, doxycycline ZYVOX susp*(PA) Authorization) hyclate, linezolid* -

Treatment Approaches for Interstitial Cystitis: Multimodality Therapy Robert J
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Treatment Approaches for Interstitial Cystitis: Multimodality Therapy Robert J. Evans, MD Moses Cone Health System, Greensboro, NC Interstitial cystitis is an increasingly common disease characterized by urgency, frequency, and pelvic pain. Its etiology is poorly understood but is likely to be multifactorial. A proposed pathophysiology describing a cascade of events, including epithelial dysfunction, mast cell activation, and neurogenic inflam- mation, is presented. Using this model, multimodality therapy regimens have been developed that treat all components of this cascade. Multimodality therapy appears more effective than single agents in the treatment of interstitial cystitis. [Rev Urol. 2002;4(suppl 1):S16–S20] © 2002 MedReviews, LLC Key words: Interstitial cystitis • Multimodality therapy • Pentosan polysulfate • Hydroxyzine hydrochloride • Amitriptyline hydrochloride nterstitial cystitis (IC) is a bladder condition that presents with a range of symptoms, including bladder pain, urinary urgency and frequency, and noc- turia. Current estimates of disease prevalence suggest that at least 1 million I 1 people in the United States are affected. The etiology remains uncertain, although a number of potential causal factors have been proposed.2–7 The variation seen in both the range of symptoms and in patients’ responses to therapy suggest that multiple factors are involved in this disease process. There remains the possibility that subgroups of patients may exist with differing etiologies. S16 VOL. 4 SUPPL. 1 2002 REVIEWS IN UROLOGY Multimodality Therapy for IC demonstrated that IC patients suffer Bladder insult from a defective GAG layer that allows urinary metabolites such as potassium to pass through the bladder wall and into the submucosal space.10 More injury Epithelial layer damage Potassium leaking through the uroepithelium causes depolarization of smooth muscles of the bladder and the pelvis, with activation of Mast cell activation and Potassium leak into sensory nerves. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Download ROCKS Patient Education Packet
Paent Educaon Checklist Following Kidney Stone Surgery The purpose of this document is to ensure a standardized method for covering all relevant paent educaon material following kidney stone surgery. It is designed to be used as a checklist while covering the key points within the accompanying ROCKS educaonal resources: Managing Pain and Urinary Symptoms following URS, Ureteral Stents: What to expect and how to manage. Managing Pain and Urinary Symptoms following Ureteroscopy Common symptoms When to call your doctor Medicaons prescribed to manage pain and reduce symptoms (please circle): Alpha‐Blockers Ancholinergics NSAIDs Opioids Other Tamsulosin (Flomax) Oxybutynin (Ditropan) Ketorolac (Toradol) Hydrocodone/acetaminophen Phenazopyridine (Pyridium) (Norco, Vicodin) Alfuzosin (Uroxatral) Tolterodine (Detrol) Ibuprofen (Motrin) Oxycodone/Paracetamol Acetaminophen (Tylenol) (Oxyconn) Other: ___________ Other:___________ Other: ___________ Other: ________________ Other: ________________ Ureteral Stent: What to expect and how to manage (See direcons for viewing our Stent Educaon Video on page 5) Was a stent placed during surgery? Yes No (If yes, complete checklist below) Defining a stent Managing stent‐related symptoms Symptoms associated with a stent Affect on daily acvies STENT REMOVAL My stent will be removed in ________ days Was the stent placed on a string? YES (circle selected opon below) NO (circle selected opon below) Paent removal | Office removal by RN Office removal | Surgical removal What can I expect aer the stent is removed? Page 1 of 5 Managing Pain and Urinary Symptoms following Ureteroscopy • You had surgery to remove or fragment your kidney stones, also known as an ureteroscopy. • After surgery, you may have some degree of pain or discomfort.