Cardiovascular System Drug Poster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Aliskiren: a Novel, Orally Active Renin Inhibitor
Review Article Aliskiren: A Novel, Orally Active Renin Inhibitor Mohamed Saleem TS, Jain A1, Tarani P1, Ravi V1, Gauthaman K1 Department of Pharmacology, Annamacharya College of Pharmacy, Rajampet, AP, 1Himalayan Pharmacy Institute, Majhitar, East Sikkim - 737 136, India ARTICLE INFO ABSTRACT Article history: Renin-angiotensin-aldosterone systems play a major role in the regulation of human homeostasis Received 01 July 2009 mechanism, which are also involved in the development of hypertension and end-organ damage Accepted 07 July 2009 through activation of angiotensin II. Inhibitors of the renin-angiotensin-aldosterone system may Available online 04 February 2010 reduce the development of end-organ damage to a greater extent than other antihypertensive Keywords: agents. Aliskiren is the first member of the new class of orally active direct renin inhibitors Aliskiren recently approved by the US Food and Drug Administration for the treatment of hypertension. Hypertension Aliskiren directly inhibiting the renin and reducing the formation of angiotensin II, which is the Renin-angiotensin-aldosterone system most effective mediator involved in the pathogenesis of cardiovascular diseases. The present Renin inhibitors review mainly focuses on the pharmacodynamics and pharmacokinetics and clinical aspects of aliskiren. In this respect, the review will improve the basic idea to understand the pharmacology of aliskiren, which is useful for the further research in cardiovascular disease. DOI: 10.4103/0975-8453.59518 Introduction inhibit renin have been available for many years but have been limited by low potency, bioavailability and duration of action. Activation of the renin-angiotensin (Ang)-aldosterone system However, a new class of nonpeptide, low molecular weight, orally [5] (RAAS) plays an important role in the development of hypertension active inhibitors has recently been developed. -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Intravesical Cocktails PBS/IC
International Painful Bladder Foundation Interstitial Cystitis/Painful Bladder Syndrome Anaesthetic intravesical cocktails 1. Anaesthetic cocktail – Robert Moldwin, MD 1:1 mixture of 0.5% Marcaine and 2% Lidocaine jelly – about 40 cc total. To this solution are added: Heparin sulphate 10,000 IU Triamcinolone 40 mg Gentamycin 80 mg or a post-procedural prophylactic antibiotic. Administration: Patients are instructed to hold the solution for about 30 minutes, then to void. When given as a diagnostic test, patients will generally sense relief of pain within 5-10 minutes. The only (rare) problems that we’ve encountered are the following: Patients may experience “rebound” pain once the solution has worn off (within 3-5 hours). This generally resolves with continued instillations. When given as therapy, we usually administer the cocktail on a weekly basis for 8-12 weeks. This is the length of time usually needed to get a prolonged response. Then, the duration between instillations is increased to q 2 weeks to q 3 weeks, etc., ultimately with the goal of discontinuance. Patients may experience urinary retention requiring catheterization. This seems to be particularly a problem in patients who appear to have pre-existing voiding dysfunction, those patients who initially present with a poor urinary flow rate, an interrupted urinary stream, etc. The urinary retention can usually be circumvented by delivering a lower total volume. 2. Marcaine with steroid cocktail – Nagendra Mishra, MD Marcaine 40 ml Heparin sulphate 10,000 IU Dexamethasone 2 cc Sodium bicarbonate 20 ml Administration: This cocktail should be held in the bladder for 20 minutes. It should be administered every 15 days for a total of 6 treatments and then as needed. -

Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: a Randomized
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6367210 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized... Article in Veterinary Surgery · May 2007 DOI: 10.1111/j.1532-950X.2007.00256.x · Source: PubMed CITATIONS READS 16 103 4 authors, including: Steven C Budsberg Mary Sarah Bergh University of Georgia Iowa State University 129 PUBLICATIONS 3,106 CITATIONS 22 PUBLICATIONS 366 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Steven C Budsberg on 11 March 2014. The user has requested enhancement of the downloaded file. Veterinary Surgery 36:234–244, 2007 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized, Placebo-Controlled Clinical Trial STEVEN C. BUDSBERG, DVM, MS, Diplomate ACVS, MARY SARAH BERGH, DVM, LISA R. REYNOLDS, BS, and HEATHER K. STREPPA, DVM, Diplomate ACVS Objective—To evaluate the efficacy of pentosan polysulfate (PPS) for improving the recovery period and mitigate the progression of osteoarthritis (OA) of the canine stifle after extracapsular stabilization of cranial cruciate ligament (CCL) injuries. Study Design—Randomized, blinded, placebo-controlled clinical trial. Animals—Dogs (n ¼ 40) with unilateral CCL instability. Methods—Each dog had an extracapsular stabilization of the stifle with or without partial men- iscectomy. Dogs were divided into 4 groups based on preoperative radiographic assessment and whether a partial meniscectomy was performed. Dogs were randomly assigned to either (3 mg/kg) PPS or placebo treatment in each group, and then injected subcutaneously weekly for 4 weeks. -

United States Patent (19) 11 4,360,518 Rovee Et Al
United States Patent (19) 11 4,360,518 Rovee et al. 45) Nov. 23, 1982 54 TOPICAL ANTI-INFLAMMATORY DRUG Primary Examiner-Stanley J. Friedman THERAPY . 57) ABSTRACT 75) Inventors: David T. Rovee, Bridgewater; John A pharmaceutical composition for topical treatment of R. Marvel; James A. Mezick, both of cutaneous disorders or disruptions characterized by East Brunswick, all of N.J. skin inflammation or hyperproliferative epidermal ac 73) Assignee: Johnson & Johnson, New Brunswick, tivity comprises the combination of a topically active N.J. anti-inflammatory corticosteroid and a non-steroidal 21 Appl. No.: 244,569 anti-inflammatory agent which is an inhibitor of prosta glandin synthetase selected from the group consisting of 22 Filed: Mar. 17, 1981 the hydratropic acid derivatives; acetylsalicylic acid; the pyrazolone derivatives; the fenamic acid deriva Related U.S. Application Data tives; the aroyl-substituted pyrroles and the substituted 60) Division of Ser. No. 64,311, Aug. 6, 1979, abandoned, arylacetohydroxamic acids in a pharmaceutically ac which is a division of Ser. No. 788,453, Apr. 20, 1977, ceptable topical vehicle. Treatment of above cutaneous Pat. No. 4,185, 100, which is a continuation-in-part of disorders may also be effected by concurrent therapy Ser. No. 685,942, May 13, 1976, abandoned. using separate applications of corticosteroid and non 51) Int. Cl. ...................... A61K 31/19; A61K 31/56 steroid. (52) U.S. Cl. ..................................... 424/240; 424/317 58) Field of Search ................................ 424/317, 240 18 Claims, No Drawings 4,360,518 1 2 Ziboh, V. A. and Snyder, D. S. 1974 Naturally occur TOPICAL ANTI-NFLAMMATORY ORUG ring and synthetic inhibitors of prostaglandin synthetase THERAPY of the skin. -
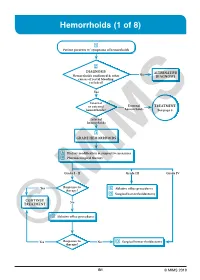
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

Supplementary Information D41586-018-05049-5
COMMENT Supplementary information to: When will clinical trials finally reflect diversity? To accompany a Comment published in Nature 557, 157–159 (2018) https://www.nature.com/articles/d41586-018-05049-5 by Todd C. Knepper & Howard L. McLeod SUPPLEMENTARY INFORMATION | NATURE | 1 Supplementary Tables Supplementary Table 1. List of drugs included in analysis Year Trade Name Non-Proprietary name Area Chemical Type Approved Gabitril tiagabine 1997 CNS 1 - New molecular entity (NME) Mirapex pramipexol 1997 CNS 1 - New molecular entity (NME) Requip ropinorole 1997 CNS 1 - New molecular entity (NME) Seroquel quetiapine 1997 CNS 1 - New molecular entity (NME) Zomig zolmitriptan 1997 CNS 1 - New molecular entity (NME) Apokyn apomorphine 2004 CNS 1 - New molecular entity (NME) Cymbalta duloxetine 2004 CNS 1 - New molecular entity (NME) Lunesta eszopiclone 2004 CNS 1 - New molecular entity (NME) Lyrica pregabalin 2004 CNS 1 - New molecular entity (NME) Prialt ziconotide 2004 CNS 1 - New molecular entity (NME) Tysabri natalizumab 2004 CNS BLA Fanapt ilioperidone 2009 CNS 1 - New molecular entity (NME) Saphris asenapine 2009 CNS 1 - New molecular entity (NME) Savella milnacipran 2009 CNS 1 - New molecular entity (NME) Amyvid florbetapir F 18 2012 CNS 1 - New molecular entity (NME) Aubagio teriflunomide 2012 CNS 1 - New molecular entity (NME) Fycompa perampanel 2012 CNS 1 - New molecular entity (NME) Belsomra suvarxant 2014 CNS 1 - New molecular entity (NME) Hetlioz tasimelteon 2014 CNS 1 - New molecular entity (NME) Movantik naloxegol 2014 CNS 1 -

Management of Joint Disease in the Sport Horse
1 MANAGEMENT OF JOINT DISEASE IN THE SPORT HORSE Management of Joint Disease in the Sport Horse C. WAYNE MCILWRAITH Colorado State University, Ft. Collins, Colorado INTRODUCTION The joint is an organ, and there are a number of ways in which traumatic damage occurs, ultimately resulting in degradation of articular cartilage. It was recognized in 1966 that articular cartilage change that accompanied osteochondral fragmentation could also be associated with concurrent traumatic damage to the attachment of the joint capsule and ligaments (Raker et al., 1966). However, there was little association made between primary disease in the synovial membrane and fibrous joint capsule and the development of osteoarthritic change in the articular cartilage until an experimental study demonstrated that cartilage degradation could occur in the horse in the absence of instability or trau- matic disruption of tissue and that loss of glycosaminoglycan (GAG) staining was associated with early morphologic breakdown at the surface of the cartilage (McIlwraith and Van Sickle, 1984). Surveys have confirmed that approximately 60% of lameness problems are related to osteoarthritis (National Animal Health Monitoring Systems, 2000; Caron and Genovese, 2003). Rapid resolution of synovitis and capsuli- tis is a critical part of the medical treatment of joint disease because of the principal role of synovitis in causing cartilage matrix breakdown. The goal of treatment of traumatic entities of the joint is twofold: (1) returning the joint to normal as quickly as possible, and (2) preventing the occurrence or reduction of the severity of osteoarthritis. In other words, treatment is intended to (1) reduce pain (lameness), and (2) minimize progression of joint deterioration.