Download This Issue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Study Is Nonmicronized Diosmin 600Mg As Effective As
Hindawi International Journal of Vascular Medicine Volume 2020, Article ID 4237204, 9 pages https://doi.org/10.1155/2020/4237204 Clinical Study Is Nonmicronized Diosmin 600mg as Effective as Micronized Diosmin 900mg plus Hesperidin 100mg on Chronic Venous Disease Symptoms? Results of a Noninferiority Study Marcio Steinbruch,1 Carlos Nunes,2 Romualdo Gama,3 Renato Kaufman,4 Gustavo Gama,5 Mendel Suchmacher Neto,6 Rafael Nigri,7 Natasha Cytrynbaum,8 Lisa Brauer Oliveira,9 Isabelle Bertaina,10 François Verrière,10 and Mauro Geller 3,6,9 1Hospital Albert Einstein (São Paulo-Brasil), R. Mauricio F Klabin 357/17, Vila Mariana, SP, Brazil 04120-020 2Instituto de Pós-Graduação Médica Carlos Chagas-Fundação Educacional Serra dos Órgãos-UNIFESO (Rio de Janeiro/Teresópolis- Brasil), Av. Alberto Torres 111, Teresópolis, RJ, Brazil 25964-004 3Fundação Educacional Serra dos Órgãos-UNIFESO (Teresópolis-Brasil), Av. Alberto Torres 111, Teresópolis, RJ, Brazil 25964-004 4Faculdade de Ciências Médicas, Universidade Estadual do Rio de Janeiro (UERJ) (Rio de Janeiro-Brazil), Av. N. Sra. De Copacapana, 664/206, Rio de Janeiro, RJ, Brazil 22050-903 5Fundação Educacional Serra dos Órgãos-UNIFESO (Teresópolis-Brasil), Rua Prefeito Sebastião Teixeira 400/504-1, Rio de Janeiro, RJ, Brazil 25953-200 6Instituto de Pós-Graduação Médica Carlos Chagas (Rio de Janeiro-Brazil), R. General Canabarro 68/902, Rio de Janeiro, RJ, Brazil 20271-200 7Department of Medicine, Rutgers New Jersey Medical School-USA, 185 S Orange Ave., Newark, NJ 07103, USA 8Hospital Universitário Pedro Ernesto, Universidade Estadual do Rio de Janeiro (UERJ) (Rio de Janeiro-Brazil), R. Hilário de Gouveia, 87/801, Rio de Janeiro, RJ, Brazil 22040-020 9Universidade Federal do Rio de Janeiro (UFRJ) (Rio de Janeiro-Brazil), Av. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Calcium Dobesilate Versus Micronised Purified Flavonoid Fraction of Diosmin in the Treatment of Chronic Venous Disease: a Randomized Prospective Study
Acta Medica Mediterranea, 2018, 34: 657 CALCIUM DOBESILATE VERSUS MICRONISED PURIFIED FLAVONOID FRACTION OF DIOSMIN IN THE TREATMENT OF CHRONIC VENOUS DISEASE: A RANDOMIZED PROSPECTIVE STUDY EMINE SEYMA DENLI YALVAC1, MURAT DEMIROĞLU2, SIDIKA GURSEL1, EBUZER AYDIN1 ¹Medeniyet University Faculty of Medicine, Department of Cardiovascular Surgery, Istanbul, Turkey - 2Medeniyet University Faculty of Medicine, Department of Department of Orthopedics and Traumatology, Istanbul, Turkey ABSTRACT Introduction: Chronic venous disease (CVD) of the lower extremities has a substantial effect on quality of life. The clinical, etiology, anatomy, pathophysiology classification (CEAP) is a frequently used classification for CVD. Patients with varicose veins are classified as CEAP class C2. Veno-active drugs (VAD) such as Calcium Dobesilate or Micronised Purified Flavonoid Fraction of Diosmin (MPFF) reduce the symptoms of pain associated with CVD. However, although the effectiveness of VAD is well established, it is controversial. The aims of the this study were to compare the efficacy of two VAD (Calcium dobesilate and MPFF in the treat- ment of CVD in view of the intended visual analogue scale (VAS) and duplex scanning (DS) changes such as measuring great saphe- nous vein (GSV) diameter. Materials and methods: A total of 24 patients who had no prior VAD treatment with CEAP class C2, with no family history of CVD were treated; 12 received calcium dobesilate and 12 received MPFF for 60 days. Comorbidities such as hypertension (HT) and diabetes mellitus (DM) were examined. Before and after treatment period, the patients were investigated with DS for diameter of GSV and pain complaint according to VAS were recorded. Results: There were no difference in treatment groups in terms of age, gender, or the comorbidities. -

Appendices Feb 2010 Inclusion of Cobalt and 2019 Fei Prohibited
APPENDIX 2 CLASSIFICATION OF PROHIBITED SUBSTANCES 1. This classification describes the types of Prohibited Substances and places same in specific categories. The list shall be published on the Club’s Notice Board. CLASS I Drugs that have the greatest pharmacological potential to affect the racing performance of a horse and which have no accepted medical use in a racehorse. These include substances which are potent stimulants of the Central Nervous System (CNS). Examples of drugs in this Class include, but are not limited to: Amphetamines, fentanyl, etorphine, cocaine, morphine, meperidine, opiates, opium derivatives, synthetic opiods, psychoactive drugs. CLASS II Drugs that have a high pharmacological potential for affecting the racing performance of a horse. These include: (i) substances which are pharmacologically active in altering the cons (ii) substances which are not generally accepted as therapeutic agents in the racing horse; and (iii) substances, some of which may have legitimate use in equine medicine but should not be found in the racing horse, such as injectable local anaesthetics. 1 Groups of drugs in this Class include, but are not limited to: (a) Opiate partial agonists, or agonistsantagonists; (b) Non-opiate psychotropic drugs, which may have stimulant, depressant, analgesic or neuroleptic effects; (c) Miscellaneous drugs which might have a stimulant effect on the CNS; (d) Drugs with prominent CNS depressant action; (e) Antidepressant and antipsychotic drugs, with or without prominent CNS stimulatory or depressant effects; (f) Muscle blocking drugs which have direct neuromuscular blocking action; (g) Local anaesthetics which have a reasonable potential for use as nerve blocking agents (except procaine); and (h) Snake venom and other biologic substances which may be used as nerve blocking agents. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group
1: Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group. A randomized, double-blind, placebo-controlled, clinical study on the efficacy and safety of calcium dobesilate in the treatment of chronic venous insufficiency. Phlebology. 2015 May 18. pii: 0268355515586097. [Epub ahead of print] PubMed PMID: 25991692. 2: Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronised purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomised trial. Int Angiol. 2015 May 14. [Epub ahead of print] PubMed PMID: 25972136. 3: Mosti G, De Maeseneer M, Cavezzi A, Parsi K, Morrison N, Nelzen O, Rabe E, Partsch H, Caggiati A, Simka M, Obermayer A, Malouf M, Flour M, Maleti O, Perrin M, Reina L, Kalodiki E, Mannello F, Rerkasem K, Cornu-Thenard A, Chi YW, Soloviy M, Bottini O, Mendyk N, Tessari L, Varghese R, Etcheverry R, Pannier F, Lugli M, Carvallo Lantz AJ, Zamboni P, Zuolo M, Godoy MF, Godoy JM, Link DP, Junger M, Scuderi A. Society for Vascular Surgery and American Venous Forum Guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. Int Angiol. 2015 Jun;34(3):202-18. Epub 2015 Apr 21. PubMed PMID: 25896614. 4: Pannier F, Rabe E. Progression in venous pathology. Phlebology. 2015 Mar;30(1 Suppl):95-7. doi: 10.1177/0268355514568847. Review. PubMed PMID: 25729075. 5: Rabe E, Pannier F. Embolization is not essential in the treatment of leg varices due to pelvic venous insufficiency. -
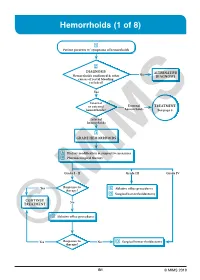
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Product Monograph
PRODUCT MONOGRAPH Sitcom LD Cream Each g contains : Euphorbia Prostrata Extract 1.0% w/w 10 mg (containing 0.315–0.825 mg total flavonoids calculated as apigenin-7-glucoside and 1.26–4.4 mg total phenolics calculated as gallic acid) and Lidocaine 3 % w/w 30 mg cream base. Cream Treatment of Haemorrhoids Manufactured By: Date of Preparation: The Madras Pharmaceuticals (05/07/2019) Old Mahabalipuram Road Karapakkam, Chennai Marketed By: Panacea Biotec Ltd. New Delhi 1 PART I: HEALTH PROFESSIONAL Page No. INFORMATION SUMMARY PRODUCT INFORMATION 4 INDICATIONS AND CLINICAL USE 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5 ADVERSE REACTIONS 7 DRUG INTERACTIONS 8 DOSAGE AND ADMINISTRATION 8 OVERDOSAGE 9 ACTION AND CLINICAL PHARMACOLOGY 9 STORAGE AND STABILITY 9 DOSAGE FORMS, COMPOSITION AND PACKAGING 9 PART II: SCIENTIFIC INFORMATION PHARMACEUTICAL INFORMATION 10 PART III: PATIENT INFORMATION 11 2 Sitcom LD Cream Each g contains : Euphorbia Prostrata Extract 1.0% w/w 10 mg (containing 0.315–0.825 mg total flavonoids calculated as apigenin-7-glucoside and 1.26–4.4 mg total phenolics calculated as gallic acid) and Lidocaine 3 % w/w 30 mg cream base. Cream Treatment of Haemorrhoids 3 PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Dosage Form / Approved Indications Administration Strength Topical Sitcom LD Cream Euphorbia Prostrata is Euphorbia Prostrata indicated for: Extract 1.0% w/w 10 mg (containing 0.315–0.825 - Treatment of Bleeding mg total flavonoids Haemorrhoids calculated as apigenin- - In post- 7-glucoside and 1.26–4.4 haemorrhoidectomy. mg total phenolics calculated as gallic acid) and Lidocaine 3 % w/w 30 mg cream base. -

PHARMACEUTICAL Antibiotics and Antibacterial
PHARMACEUTICAL Antibiotics and Antibacterial Active Ingredient Form Strength Pack Type Packing Style Amoxicillin Sodium Dry Powder Injections 250mg, 500mg, 1000 mg Vial 1's Amoxicillin Suspension 125mg/5ml, 250mg/5ml Bottle 30ml, 60ml Amoxicillin Dispersible Tablet 125mg, 250mg Strip 10's Amoxicillin Trihydrate Tablet 1000mg Blister 10's Amoxicillin + Clavulanate Potassium Injection 500mg + 100mg, 1g + 200mg Vial 10ml, 20ml Amoxicillin + Clavulanate Potassium Suspension 200mg + 28.5mg Bottle 30ml Amoxicillin + clavulanic Acid Tablets 250mg + 125mg, 500mg + 125mg Strip , Alu-Alu 6's, 10's Amoxicillin + Cloxacillin Capsules 250mg + 250mg, 500mg + 500mg Blister 10's Amoxicillin + Cloxacillin Dry Powder Injections 250mg + 250mg Vial 1's Amoxiciilin + Cloxacillin + Lactic Acid Tablet 250 mg + 250 mg + 60 million Tablet 10's Bacillus Spores, 250 + 250 + 120 million Spores Amoxicillin + Flucloxacillin Capsules 250mg + 250mg Strip 10's Azithromycin Tablets 250mg, 500mg Blister 6's, 3's Azithromycin + Fluconazole + Secnidazole Tablet 1gm + 150mg + 1gm Strip 1 + 1 + 2 Betamethasone Valerate + Neomycin Cream 0.1% + 0.5% Tube 10g Sulphate Beclomethasone + Phenylephrine Hcl + Cream 0.025% + 0.10% + 2.5% + 0.1% Tube 10g Lignocaine Hcl + Chlorocresol Betamethasone Valerate Lotion 0.10% Bottle 30ml Betamethasone Injection 4mg/ml Ampoule 1ml Bethamethasone Opthalmic Solution 0.1% w/v Bottle 5ml Betamethasone Valerate Cream 0.1%w/w Tube 15 g Bethamethasone + Gentamicin Cream 0.1% + 0.05% Tube 10 g Betamethasone Sodium Phosphate Tablet 0.5mg Blister 10s -

DATA SUPPLEMENT Safety and Efficacy of Combination Nivolumab
DATA SUPPLEMENT Safety and Efficacy of Combination Nivolumab Plus Ipilimumab in Patients with Advanced Melanoma: Results from a North American Expanded Access Program (CheckMate 218) F. Stephen Hodi, Paul B. Chapman, Mario Sznol, Christopher D. Lao, Rene Gonzalez, Michael Smylie, Gregory A. Daniels, John A. Thompson, Ragini Kudchadkar, William Sharfman, Michael Atkins, David R. Spigel, Anna Pavlick, Jose Monzon, Kevin B. Kim, Scott Ernst, Nikhil I. Khushalani, Wim van Dijck, Maurice Lobo, David Hogg 1 Supplementary Tables Table S1: Summary of adverse events reported between first dose and 30 days after last dose of the EAP therapy that required immune modulating medications in ≥1% of patients Nivolumab plus ipilimumab (N=754) Any grade, Grade 3–4, N (%)a N (%) Any adverse event 600 (80) 332 (44) Diarrhea 132 (18) 52 (7) Maculopapular rash 115 (15) 22 (3) Colitis 76 (10) 57 (8) Increased alanine aminotransferase 72 (10) 47 (6) Increased aspartate aminotransferase 58 (8) 32 (4) Rash 52 (7) 3 (<1) Pruritus 47 (6) 3 (<1) Pneumonitis 39 (5) 9 (1) Hypophysitis 38 (5) 5 (1) Autoimmune hepatitis 37 (5) 28 (4) Pruritic rash 29 (4) 0 Generalized pruritus 22 (3) 3 (<1) Nausea 20 (3) 5 (1) Adrenal insufficiency 18 (2) 3 (<1) Generalized rash 15 (2) 5 (1) Fatigue 14 (2) 3 (<1) Malignant neoplasm progression 12 (2) 11 (1) Arthralgia 12 (2) 2 (<1) Vomiting 12 (2) 2 (<1) Uveitis 11 (1) 1 (<1) Headache 11 (1) 1 (<1) increased transaminases 11 (1) 8 (1) Macular rash 11 (1) 2 (<1) Pyrexia 10 (1) 1 (<1) Increased lipase 10 (1) 9 (1) Acute kidney injury 9 (1) 6 (1) Hepatitis 9 (1) 6 (1) Cough 9 (1) 0 Acneiform dermatitis 9 (1) 0 Abdominal pain 8 (1) 2 (<1) aOne grade 5 adverse event (due to malignant neoplasm progression) was reported. -

Malta Medicines List 25 7 07
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Zovirax™ Suspension 200mg/5ml Oral suspension Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4gAntiviral J05AB01 PoM Zovirax® 800mg Aciclovir Aciclovir Medovir 800 800mg Tablet Virucid 800 Virucid 400 400mg Tablet Zovirax® Cream 5% w/w Cream Refer to PIL Antiviral D06BB03 PoM Medovir Zovirax® Eye Ointment 3% w/w Eye ointment Refer to PIL Antiviral S01AD03 PoM Neotigason® 10mg Retinoid - Acitretin Capsule 35mg D05BB02 PoM Neotigason® 25mg Antipsoriatic Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Antidiarrhoeal and Activated Charcoal Biocarbon® 0.25g Tablet 5g A07BA01 OTC Antiflatulent Dentinox® Infant Colic 42mg/ml Oral suspension Drops Antifoaming agent - Activated Dimethicone Aero-OM® 100mg/ml Oral drops emulsion Refer to PILPreparation for colic A02X OTC or wind pain Aero-OM® 40mg Tablet Activated dimeticone, Antacid and Asilone Antacid Tablets 270mg 500mg Tablet Refer to PIL A02AB10 OTC Dried aluminium hydroxide Antiflatulent