Clinical Study Is Nonmicronized Diosmin 600Mg As Effective As
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Benefits of Flavonoids in Diabetic Retinopathy
nutrients Review The Benefits of Flavonoids in Diabetic Retinopathy 1, 1, 2,3,4,5 1,2,3,4, Ana L. Matos y, Diogo F. Bruno y, António F. Ambrósio and Paulo F. Santos * 1 Department of Life Sciences, University of Coimbra, Calçada Martim de Freitas, 3000-456 Coimbra, Portugal; [email protected] (A.L.M.); [email protected] (D.F.B.) 2 Coimbra Institute for Clinical and Biomedical Research (iCBR), Faculty of Medicine, University of Coimbra, 3000-548 Coimbra, Portugal; [email protected] 3 Center for Innovative Biomedicine and Biotechnology (CIBB), University of Coimbra, 3000-548 Coimbra, Portugal 4 Clinical Academic Center of Coimbra (CACC), 3004-561 Coimbra, Portugal 5 Association for Innovation and Biomedical Research on Light and Image (AIBILI), 3000-548 Coimbra, Portugal * Correspondence: [email protected]; Tel.: +351-239-240-762 These authors contributed equally to the work. y Received: 10 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: Diabetic retinopathy (DR), one of the most common complications of diabetes, is the leading cause of legal blindness among adults of working age in developed countries. After 20 years of diabetes, almost all patients suffering from type I diabetes mellitus and about 60% of type II diabetics have DR. Several studies have tried to identify drugs and therapies to treat DR though little attention has been given to flavonoids, one type of polyphenols, which can be found in high levels mainly in fruits and vegetables, but also in other foods such as grains, cocoa, green tea or even in red wine. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Chondroprotective Agents
Europaisches Patentamt J European Patent Office © Publication number: 0 633 022 A2 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 94109872.5 © Int. CI.6: A61K 31/365, A61 K 31/70 @ Date of filing: 27.06.94 © Priority: 09.07.93 JP 194182/93 Saitama 350-02 (JP) Inventor: Niimura, Koichi @ Date of publication of application: Rune Warabi 1-718, 11.01.95 Bulletin 95/02 1-17-30, Chuo Warabi-shi, 0 Designated Contracting States: Saitama 335 (JP) CH DE FR GB IT LI SE Inventor: Umekawa, Kiyonori 5-4-309, Mihama © Applicant: KUREHA CHEMICAL INDUSTRY CO., Urayasu-shi, LTD. Chiba 279 (JP) 9-11, Horidome-cho, 1-chome Nihonbashi Chuo-ku © Representative: Minderop, Ralph H. Dr. rer.nat. Tokyo 103 (JP) et al Cohausz & Florack @ Inventor: Watanabe, Koju Patentanwalte 2-5-7, Tsurumai Bergiusstrasse 2 b Sakado-shi, D-30655 Hannover (DE) © Chondroprotective agents. © A chondroprotective agent comprising a flavonoid compound of the general formula (I): (I) CM < CM CM wherein R1 to R9 are, independently, a hydrogen atom, hydroxyl group, or methoxyl group and X is a single bond or a double bond, or a stereoisomer thereof, or a naturally occurring glycoside thereof is disclosed. The 00 00 above compound strongly inhibits proteoglycan depletion from the chondrocyte matrix and exhibits a function to (Q protect cartilage, and thus, is extremely effective for the treatment of arthropathy. Rank Xerox (UK) Business Services (3. 10/3.09/3.3.4) EP 0 633 022 A2 BACKGROUND OF THE INVENTION 1 . Field of the Invention 5 The present invention relates to an agent for protecting cartilage, i.e., a chondroprotective agent, more particularly, a chondroprotective agent containing a flavonoid compound or a stereoisomer thereof, or a naturally occurring glycoside thereof. -

Calcium Dobesilate Versus Micronised Purified Flavonoid Fraction of Diosmin in the Treatment of Chronic Venous Disease: a Randomized Prospective Study
Acta Medica Mediterranea, 2018, 34: 657 CALCIUM DOBESILATE VERSUS MICRONISED PURIFIED FLAVONOID FRACTION OF DIOSMIN IN THE TREATMENT OF CHRONIC VENOUS DISEASE: A RANDOMIZED PROSPECTIVE STUDY EMINE SEYMA DENLI YALVAC1, MURAT DEMIROĞLU2, SIDIKA GURSEL1, EBUZER AYDIN1 ¹Medeniyet University Faculty of Medicine, Department of Cardiovascular Surgery, Istanbul, Turkey - 2Medeniyet University Faculty of Medicine, Department of Department of Orthopedics and Traumatology, Istanbul, Turkey ABSTRACT Introduction: Chronic venous disease (CVD) of the lower extremities has a substantial effect on quality of life. The clinical, etiology, anatomy, pathophysiology classification (CEAP) is a frequently used classification for CVD. Patients with varicose veins are classified as CEAP class C2. Veno-active drugs (VAD) such as Calcium Dobesilate or Micronised Purified Flavonoid Fraction of Diosmin (MPFF) reduce the symptoms of pain associated with CVD. However, although the effectiveness of VAD is well established, it is controversial. The aims of the this study were to compare the efficacy of two VAD (Calcium dobesilate and MPFF in the treat- ment of CVD in view of the intended visual analogue scale (VAS) and duplex scanning (DS) changes such as measuring great saphe- nous vein (GSV) diameter. Materials and methods: A total of 24 patients who had no prior VAD treatment with CEAP class C2, with no family history of CVD were treated; 12 received calcium dobesilate and 12 received MPFF for 60 days. Comorbidities such as hypertension (HT) and diabetes mellitus (DM) were examined. Before and after treatment period, the patients were investigated with DS for diameter of GSV and pain complaint according to VAS were recorded. Results: There were no difference in treatment groups in terms of age, gender, or the comorbidities. -

Appendices Feb 2010 Inclusion of Cobalt and 2019 Fei Prohibited
APPENDIX 2 CLASSIFICATION OF PROHIBITED SUBSTANCES 1. This classification describes the types of Prohibited Substances and places same in specific categories. The list shall be published on the Club’s Notice Board. CLASS I Drugs that have the greatest pharmacological potential to affect the racing performance of a horse and which have no accepted medical use in a racehorse. These include substances which are potent stimulants of the Central Nervous System (CNS). Examples of drugs in this Class include, but are not limited to: Amphetamines, fentanyl, etorphine, cocaine, morphine, meperidine, opiates, opium derivatives, synthetic opiods, psychoactive drugs. CLASS II Drugs that have a high pharmacological potential for affecting the racing performance of a horse. These include: (i) substances which are pharmacologically active in altering the cons (ii) substances which are not generally accepted as therapeutic agents in the racing horse; and (iii) substances, some of which may have legitimate use in equine medicine but should not be found in the racing horse, such as injectable local anaesthetics. 1 Groups of drugs in this Class include, but are not limited to: (a) Opiate partial agonists, or agonistsantagonists; (b) Non-opiate psychotropic drugs, which may have stimulant, depressant, analgesic or neuroleptic effects; (c) Miscellaneous drugs which might have a stimulant effect on the CNS; (d) Drugs with prominent CNS depressant action; (e) Antidepressant and antipsychotic drugs, with or without prominent CNS stimulatory or depressant effects; (f) Muscle blocking drugs which have direct neuromuscular blocking action; (g) Local anaesthetics which have a reasonable potential for use as nerve blocking agents (except procaine); and (h) Snake venom and other biologic substances which may be used as nerve blocking agents. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group
1: Rabe E, Ballarini S, Lehr L; Doxium EDX09/01 Study Group. A randomized, double-blind, placebo-controlled, clinical study on the efficacy and safety of calcium dobesilate in the treatment of chronic venous insufficiency. Phlebology. 2015 May 18. pii: 0268355515586097. [Epub ahead of print] PubMed PMID: 25991692. 2: Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronised purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomised trial. Int Angiol. 2015 May 14. [Epub ahead of print] PubMed PMID: 25972136. 3: Mosti G, De Maeseneer M, Cavezzi A, Parsi K, Morrison N, Nelzen O, Rabe E, Partsch H, Caggiati A, Simka M, Obermayer A, Malouf M, Flour M, Maleti O, Perrin M, Reina L, Kalodiki E, Mannello F, Rerkasem K, Cornu-Thenard A, Chi YW, Soloviy M, Bottini O, Mendyk N, Tessari L, Varghese R, Etcheverry R, Pannier F, Lugli M, Carvallo Lantz AJ, Zamboni P, Zuolo M, Godoy MF, Godoy JM, Link DP, Junger M, Scuderi A. Society for Vascular Surgery and American Venous Forum Guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. Int Angiol. 2015 Jun;34(3):202-18. Epub 2015 Apr 21. PubMed PMID: 25896614. 4: Pannier F, Rabe E. Progression in venous pathology. Phlebology. 2015 Mar;30(1 Suppl):95-7. doi: 10.1177/0268355514568847. Review. PubMed PMID: 25729075. 5: Rabe E, Pannier F. Embolization is not essential in the treatment of leg varices due to pelvic venous insufficiency. -
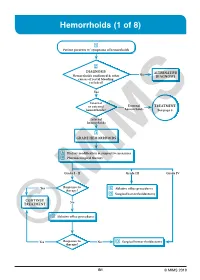
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

16441-16455 Page 16441 S
S. Umamaheswari*et al. /International Journal of Pharmacy & Technology ISSN: 0975-766X CODEN: IJPTFI Available Online through Research Article www.ijptonline.com EFFECT OF FLAVONOIDS DIOSMIN, MORIN AND CHRYSIN ON CHANG LIVER CELL LINE K.S. Sridevi Sangeetha1, S. Umamaheswari1*, C. Uma Maheswara Reddy1, S. Narayana kalkura2 1 Department of Pharmacology, Faculty of Pharmacy, Sri Ramachandra University, Porur, Chennai 2Crystal Growth Centre, Anna University, Guindy, Chennai. Email: [email protected] Received on 06-08-2016 Accepted on 27-08-2016 Abstract Objective: To evaluate the effect of selected flavonoids diosmin, morin and chrysin on chang cell (normal human liver cells) line by using cell viability assay. Methods: The cell viability assay on chang cell was determined using MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5- diphenyltetrazolium bromide) assay. Diosmin, morin and chrysin were subjected in the concentration of 1.625 µM, 3.125 µM, 6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM and 500 µM respectively. Results: The cytoprotective activity by MTT method showed that the IC 50 value of diosmin, morin and chrysin was 101.91 µM, 14.62 µM and 70.00 µM respectively. Conclusion: Out of the three flavonoids, diosmin and chrysin were proven to have very good cytoprotective activity against Chang cell line. The order of activity was found to be Disomin > Chrysin > Morin. Key words: Flavonoid, Chang cell line, Diosmin, Morin, Chrysin Introduction Plant produces different types of secondary metabolites and one such group is flavonoid. They are polyphenolic in nature and present in different part of the plant like leaves, flowers, fruits, vegetable etc. -

PHARMACEUTICAL Antibiotics and Antibacterial
PHARMACEUTICAL Antibiotics and Antibacterial Active Ingredient Form Strength Pack Type Packing Style Amoxicillin Sodium Dry Powder Injections 250mg, 500mg, 1000 mg Vial 1's Amoxicillin Suspension 125mg/5ml, 250mg/5ml Bottle 30ml, 60ml Amoxicillin Dispersible Tablet 125mg, 250mg Strip 10's Amoxicillin Trihydrate Tablet 1000mg Blister 10's Amoxicillin + Clavulanate Potassium Injection 500mg + 100mg, 1g + 200mg Vial 10ml, 20ml Amoxicillin + Clavulanate Potassium Suspension 200mg + 28.5mg Bottle 30ml Amoxicillin + clavulanic Acid Tablets 250mg + 125mg, 500mg + 125mg Strip , Alu-Alu 6's, 10's Amoxicillin + Cloxacillin Capsules 250mg + 250mg, 500mg + 500mg Blister 10's Amoxicillin + Cloxacillin Dry Powder Injections 250mg + 250mg Vial 1's Amoxiciilin + Cloxacillin + Lactic Acid Tablet 250 mg + 250 mg + 60 million Tablet 10's Bacillus Spores, 250 + 250 + 120 million Spores Amoxicillin + Flucloxacillin Capsules 250mg + 250mg Strip 10's Azithromycin Tablets 250mg, 500mg Blister 6's, 3's Azithromycin + Fluconazole + Secnidazole Tablet 1gm + 150mg + 1gm Strip 1 + 1 + 2 Betamethasone Valerate + Neomycin Cream 0.1% + 0.5% Tube 10g Sulphate Beclomethasone + Phenylephrine Hcl + Cream 0.025% + 0.10% + 2.5% + 0.1% Tube 10g Lignocaine Hcl + Chlorocresol Betamethasone Valerate Lotion 0.10% Bottle 30ml Betamethasone Injection 4mg/ml Ampoule 1ml Bethamethasone Opthalmic Solution 0.1% w/v Bottle 5ml Betamethasone Valerate Cream 0.1%w/w Tube 15 g Bethamethasone + Gentamicin Cream 0.1% + 0.05% Tube 10 g Betamethasone Sodium Phosphate Tablet 0.5mg Blister 10s -

Download This Issue
UIP Chapter Meeting - August 25th -27th 2019, Krakow – Poland Contents Foreword 83 UIP Chapter Meeting August 25th -27th 2019, Krakow – Poland 85 Charing Cross International Symposium - Vascular & Endovascular Challenges Update April 15th-18th 2019, London – UK 191 81 Vol 26. No. 3. 2019 Medical Reporters’ Academy The reports from the UIP chapter meeting and Charing cross international symposium were prepared by the following members of the Medical Reporters’ Academy: Roman BREDIKHIN (Russia) Kirill LOBASTOV (Russia) Daniela MASTROIACOVO (Italy) Mustafa SIRLAK (Turkey) Stanislava TZANEVA (Austria) and chaired by: Andrew NICOLAIDES (Cyprus) 82 UIP Chapter Meeting - August 25th -27th 2019, Krakow – Poland Foreword The Medical Reporters’ Academy was co-founded in 1994 as a joint initiative by clinical and research venous disease specialists and Servier; with the main objective to develop an international group of young specialists with a core interest in venous disease drawn from various fields including dermatology, vascular surgery, angiology and/or phlebology. Each year the Medical Reporters’ Academy members are invited by Servier, to cover one of the most important international congresses for venous disease specialists. This year, the UIP Chapter Meeting, which was held in Krakow, Poland on August 25th- 27th, 2019 and the Charing Cross International Symposium: Vascular & Endovascular Challenges Update which was held in London, United Kingdom on April 15th - 18th, 2019 were selected because these congresses involved renowned international and national venous experts and young health care professionals providing a great opportunity to exchange ideas, explore strategies for vein care and discuss the latest trends & innovations in the field. Together with Andrew Nicolaides, the chairman of the group, the academy members explored the program of the congress, selected the events and presentations likely to present breakthroughs or new findings to attend, and wrote short reports. -

Electrochemical Behaviour of Flavonoids on a Surface
44 Biomed. Papers 149 (Supplement 1), 2005 ELECTROCHEMICAL BEHAVIOUR 3/ OF FLAVONOIDS ON A SURFACE / / OF A CARBON PASTE ELECTRODE 2 4 8 / Pavel Hanuštiaka, Radka Mikelováa, O 1 5/ a, b c c 7 David Potěšil , Petr Hodek , Marie Stiborová , 1 2 René Kizeka 6/ 6 a Department of Chemistry and Biochemistry, Faculty 3 of Agronomy, Mendel University of Agriculture and 4 5 Forestry, Zemědělská 1, CZ-613 00 Brno, Czech Republic O b Department of Analytical Chemistry, Faculty of Science, Fig. 1. Flavonoid structure made of two benzene rings Masaryk University, Kotlářská 37, CZ-611 37 Brno, linked by heterocyclic pyran. Czech Republic c Department of Biochemistry, Faculty of Science, Charles University, Albertov 2030, CZ-128 40 Prague, of health and could significantly support a physiological Czech Republic capacity or decrease a possibility of a disease appearing3. e-mail: [email protected] Different groups of metabolites such as phenolic acids, lignans, phytosterols, carotenoids, glucosinolates and Key words: Flavonoids/Antioxidant/Rutin/Quercetin/ also flavonoids influence human health as described Quercitrin/Diosmin/Chrysin/Carbon paste electrode/ above2. Flavonoids comprise a wide-ranging group of Square wave voltammetry plant phenols. Up to now, more than 4,000 of flavonoid compounds are known and new ones have been still dis- covering. Flavonoids are derived from heterocyclic oxy- ABSTRACT gen compound, flavan, which is formed by two benzene rings linked by heterocyclic pyran (Fig. 1). All three rings Recent papers discuss relation of flavonoid compounds could be substituted by hydroxy- or methoxy-groups and and tumour diseases. In addition it is impossible to say particular derivates differs only in degree of substitution that effect of flavonoids is positive or negative without and oxidation.