Prescription Drug Formulary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMMISSION DE LA TRANSPARENCE Avis 5 Septembre 2018
COMMISSION DE LA TRANSPARENCE Avis 5 septembre 2018 Date d’examen par la Commission : 20 juin 2018 L’avis de la commission de la Transparence adopté le 11 juillet 2018 a fait l’objet d’une audition le 5 septembre 2018. letermovir PREVYMIS 240 mg, comprimés pelliculés Boîte de 28 (CIP : 34009 301 272 3 0) PREVYMIS 240 mg, solution à diluer pour perfusion Boite de 1 (CIP : 34009 301 272 5 4) PREVYMIS 480 mg, comprimés pelliculés Boîte de 28 (CIP : 34009 301 272 4 7) PREVYMIS 480 mg, solution à diluer pour perfusion Boite de 1 (CIP : 34009 301 272 6 1) Laboratoire MSD France Code ATC J05AX18 (Antiviral) Motif de l’examen Inscription Sécurité Sociale (CSS L.162-17) pour les formes comprimés uniquement Listes concernées Collectivités (CSP L.5123-2) pour l’ensemble des présentations « PREVYMIS est indiqué dans la prophylaxie de la réactivation du cytomégalovirus (CMV) et de la maladie à CMV chez les adultes séropositifs au CMV receveurs [R+] d’une greffe allogénique de cellules Indication concernée souches hématopoïétiques (GCSH). Il convient de tenir compte des recommandations officielles concernant l’utilisation appropriée des agents antiviraux. » HAS - Direction de l'Evaluation Médicale, Economique et de Santé Publique 1/18 Avis3 SMR Important Compte tenu : - de la démonstration de l’efficacité de PREVYMIS en prophylaxie de la réactivation du CMV par rapport au placebo et notamment la diminution des instaurations de traitements préemptifs exposant les patients à une toxicité hématologique ou rénale importante, - de l’absence d’impact démontré -

Subtherapeutic Posaconazole Prophylaxis in a Gastric Bypass Patient Following Hematopoietic Stem Cell Transplantation
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” CASE REPORT Subtherapeutic posaconazole prophylaxis in a gastric bypass patient following hematopoietic stem cell transplantation Emily A. Highsmith, PharmD, BCCCP, Department of Pharmacy, University Purpose. A case of invasive fungal infections (IFIs) with subtherapeutic of Texas MD Anderson Cancer Center, Houston, TX, USA posaconazole prophylaxis in a gastric bypass patient following hemato- poietic stem cell transplantation (HSCT) is reported. Downloaded from https://academic.oup.com/ajhp/article/78/14/1282/6245082 by BINASSS user on 15 July 2021 Vi P. Doan, PharmD, BCOP, Department of Pharmacy, University of Texas MD Anderson Cancer Center, Summary. A 52-year-old malnourished male with a medical history of Houston, TX, USA Roux-en-Y gastric bypass for obesity developed acute myelogenous leu- Todd W. Canada, PharmD, BCNSP, kemia and underwent allogeneic HSCT approximately 17 months later. He BCCCP, FASHP, FTSHP, FASPEN, was admitted 1 month after HSCT for failure to thrive and initiated on par- Department of Pharmacy, University of Texas MD Anderson Cancer Center, enteral nutrition due to worsening diarrhea and suspected gastrointestinal Houston, TX, USA graft-versus-host disease (GI GVHD). During admission, the patient was continued on daily oral posaconazole for antifungal prophylaxis and was found to have subtherapeutic posaconazole and deficient vitamin levels, likely secondary to his gastrojejunostomy and increased gastric transit time. The oral posaconazole was altered to twice-daily dosing in an effort to increase serum drug levels and prevent IFIs. Conclusion. Patients with a history of gastric bypass are at increased risk for malabsorption of oral posaconazole and nutrients, especially following HSCT with suspected GI GVHD. -

Xifaxan® (Rifaximin)
Wednesday, October 14, 2015 4 p.m. Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for Board Meeting – October 14, 2015 DATE: October 1, 2015 NOTE: The DUR Board will meet at 4:00 p.m. The meeting will be held at 4345 N Lincoln Blvd. Enclosed are the following items related to the October meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Action Item – Vote on 2016 Meeting Dates – Appendix B Update on Medication Coverage Authorization Unit/Bowel Preparation Medication Post-Educational Mailing – Appendix C Action Item – Vote to Prior Authorize Tykerb® (Lapatinib), Halaven® (Eribulin), Ixempra® (Ixabepilone), Kadcyla® (Ado-Trastuzumab), Afinitor® (Everolimus), & Perjeta® (Pertuzumab) – Appendix D Action Item – Vote to Prior Authorize Orkambi™ (Lumacaftor/Ivacaftor) – Appendix E Action Item – Vote to Prior Authorize Savaysa® (Edoxaban) – Appendix F Action Item – Vote to Prior Authorize Epanova® (Omega-3-Carboxylic Acids), Praluent® (Alirocumab), & Repatha™ (Evolocumab) – Appendix G Annual Review of Constipation and Diarrhea Medications and 30-Day Notice to Prior Authorize Movantik™ (Naloxegol), Viberzi™ (Eluxadoline), & Xifaxan® (Rifaximin) – Appendix H 30-Day Notice to Prior Authorize Daraprim® (Pyrimethamine) – Appendix I Annual Review of Allergy Immunotherapies and 30-Day Notice to Prior Authorize Oralair® (Sweet Vernal, Orchard, Perennial Rye, Timothy, & Kentucky Blue Grass Mixed Pollens Allergen Extract) – Appendix J Annual Review of Non-Steroidal Anti-Inflammatory Drugs and 30-Day Notice to Prior Authorize Dyloject™ (Diclofenac Sodium) – Appendix K ORI-4403 • P.O. -

R&D Briefing 76
The Impact of Recent Regulatory Developments on the Mexican Therapeutic Landscape Access to innovative medicines is key to El acceso a medicamentos innovadores es clave para improving overall population health, reducing mejorar la salud de toda la población, para reducir los hospitalisation time and decreasing morbidity and tiempos de hospitalización, la morbilidad y la mortalidad mortality. An efficient regulatory process can be de un país. Un proceso regulatorio eficiente tiene un reflected in measurable positive health impacts; impacto positivo medible en la salud, y por el contrario, conversely, activities that slow or impede acciones que retrasan o impiden la eficiencia regulatoria y regulatory efficiency and predictability can be su predictibilidad pueden ser perjudiciales. La parálisis detrimental. Recent developments in the Mexican reciente del Sistema regulatorio mexicano respecto a la regulatory system for the assessments of evaluación de nuevos medicamentos innovadores innovative new products have had a negative conlleva un impacto negativo en la salud de la población impact on Mexican public health. mexicana. This Briefing addresses the impact of suspending Este informe analiza el impacto de la suspensión de las the activities of the New Molecules Committee actividades del Comité de Moléculas Nuevas (NMC, por (NMC) on the Mexican therapeutic landscape. sus siglas en inglés) sobre el horizonte terapéutico de First, we compared the way that “new medicines” México. En primer lugar, comparamos la definición de are defined within the context of the Mexican nuevos medicamentos según el contexto regulatorio regulatory system, with definitions used by mexicano con las definiciones adoptadas por otras comparable regulators and health organisations. agencias reguladoras u organizaciones de salud del We have also investigated the extent to which mundo. -

11-2018 BMT Pharmacists Johnson FINAL 2 12 18
2018 BMT Pharmacists Conference Infection Prophylaxis in the HCT patient Melissa D. Johnson, PharmD, AAHIVP Associate Professor of Medicine Duke University Medical Center Liaison Clinical Pharmacist Duke Antimicrobial Stewardship Outreach Network Durham, North Carolina [email protected] @idpharmacist Disclosures • Research grants: Merck, Charles River Laboratories Objectives • Assess the factors influencing risk of infection in hematopoietic cell transplant recipients • Compare and contrast current, recently approved and emerging antimicrobials for prophylaxis in hematopoietic cell transplant recipients • Discuss recent studies evaluating antimicrobials for prophylaxis in hematopoietic cell transplant recipients • Identify important monitoring parameters and supportive strategies for prophylaxis in hematopoietic cell transplant recipients Hematopoietic Cell Transplantation Transplant Variables and Infection Risk • Underlying disease Pre-Tx Stem cells • Type of transplant Therapy +/- GVHD Autologous, Allogeneic • HLA matching Conditioning • Conditioning regimen Engraftment • Source of stem cells • Graft manipulation • Graft versus host disease • Donor & recipient age Bacterial Viruses Fungal INFECTIOUS PATHOGENS Respiratory Pneumocystis Viruses Other Case Question 1 • 37-year-old male with AML Initial presentation: • 45,000 WBC, normal cytogenetics, FLT3/ITD genes (+), NPM1 (-) Treatment plan: Standard induction followed by allogeneic hematopoietic peripheral blood stem-cell transplant • Which of the following pathogens is he at greatest -

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -
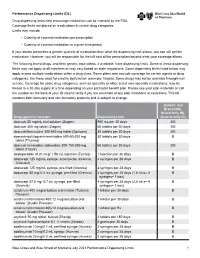
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Hinge Region in DNA Packaging Terminase Pul15 of Herpes Simplex Virus: a Potential Allosteric Target for Antiviral Drugs
Louisiana State University LSU Digital Commons Faculty Publications School of Renewable Natural Resources 10-1-2019 Hinge Region in DNA Packaging Terminase pUL15 of Herpes Simplex Virus: A Potential Allosteric Target for Antiviral Drugs Lana F. Thaljeh Louisiana State Univ, Dept Biol Sci, [email protected] J. Ainsley Rothschild Louisiana State Univ, Div Elect & Comp Engn, [email protected] Misagh Naderi Louisiana State Univ, Dept Biol Sci, [email protected] Lyndon M. Coghill Louisiana State Univ, Dept Biol Sci, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/agrnr_pubs Part of the Biology Commons Recommended Citation Thaljeh, Lana F.; Rothschild, J. Ainsley; Naderi, Misagh; and Coghill, Lyndon M., "Hinge Region in DNA Packaging Terminase pUL15 of Herpes Simplex Virus: A Potential Allosteric Target for Antiviral Drugs" (2019). Faculty Publications. 4. https://digitalcommons.lsu.edu/agrnr_pubs/4 This Article is brought to you for free and open access by the School of Renewable Natural Resources at LSU Digital Commons. It has been accepted for inclusion in Faculty Publications by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. biomolecules Article Hinge Region in DNA Packaging Terminase pUL15 of Herpes Simplex Virus: A Potential Allosteric Target for Antiviral Drugs Lana F. Thaljeh 1, J. Ainsley Rothschild 1, Misagh Naderi 1, Lyndon M. Coghill 1,2 , Jeremy M. Brown 1 and Michal Brylinski 1,2,* 1 Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA; [email protected] (L.F.T.); [email protected] (J.A.R.); [email protected] (M.N.); [email protected] (L.M.C.); [email protected] (J.M.B.) 2 Center for Computation & Technology, Louisiana State University, Baton Rouge, LA 70803, USA * Correspondence: [email protected]; Tel.: +225-578-2791; Fax: +225-578-2597 Received: 10 September 2019; Accepted: 8 October 2019; Published: 12 October 2019 Abstract: Approximately 80% of adults are infected with a member of the herpesviridae family. -

Where Do We Stand After Decades of Studying Human Cytomegalovirus?
microorganisms Review Where do we Stand after Decades of Studying Human Cytomegalovirus? 1, 2, 1 1 Francesca Gugliesi y, Alessandra Coscia y, Gloria Griffante , Ganna Galitska , Selina Pasquero 1, Camilla Albano 1 and Matteo Biolatti 1,* 1 Laboratory of Pathogenesis of Viral Infections, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] (F.G.); gloria.griff[email protected] (G.G.); [email protected] (G.G.); [email protected] (S.P.); [email protected] (C.A.) 2 Complex Structure Neonatology Unit, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 March 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Human cytomegalovirus (HCMV), a linear double-stranded DNA betaherpesvirus belonging to the family of Herpesviridae, is characterized by widespread seroprevalence, ranging between 56% and 94%, strictly dependent on the socioeconomic background of the country being considered. Typically, HCMV causes asymptomatic infection in the immunocompetent population, while in immunocompromised individuals or when transmitted vertically from the mother to the fetus it leads to systemic disease with severe complications and high mortality rate. Following primary infection, HCMV establishes a state of latency primarily in myeloid cells, from which it can be reactivated by various inflammatory stimuli. Several studies have shown that HCMV, despite being a DNA virus, is highly prone to genetic variability that strongly influences its replication and dissemination rates as well as cellular tropism. In this scenario, the few currently available drugs for the treatment of HCMV infections are characterized by high toxicity, poor oral bioavailability, and emerging resistance. -

List of Drugs Not Repackaged by Safecor Health
Drugs Not Repackaged by Safecor Health The following tables list specific medications that are not repackaged by Safecor Health due to regulatory restrictions or specific manufacturer requirements. The items not repackaged are alphabetically listed below, both by brand name (table 1) and generic name (table 2). Please note: Safecor Health cannot repackage any beta lactam antibiotics (such as penicillins, amoxicillin and cephalosporins) or potent chemotherapeutic agents. Also, due to FDA restrictions, we cannot repackage half- or quarter-tabs, compounded or diluted drugs, powders, and ointments or creams. Safecor Health can repackage most hazardous drugs on the NIOSH list. Contact us for a complete list of hazardous drugs repackaged by Safecor Health. Table 1. Do Not Repackage Drugs Sorted Alphabetically by Brand Name Brand Name(s) Generic Name(s) Reason Item Cannot Be Repackaged Adrucil Fluorouracil Potent chemotherapy agent Aspirin and Extended-Release Specific manufacturer recommendations for very Aggrenox Dipyridamole limited expiration dating Albenza Albendazole Cost per dose prohibitive Alkeran Melphalan Potent chemotherapy agent Augmentin Amoxicillin and Clavulanate Potassium Safecor Health does not repackage this drug class Manufacturer states, "dispense in original container," Belsomra Suvorexant on the drug label Manufacturer states, "dispense in original container," Biktarvy Bictegravir, Emtricitabine and Tenofovir Alafenamide on the drug label Bion Tears Dextran, Hypromellose Ophthalmic Drops Sterile and unpreserved CeeNU Lomustine -

Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: a Case Series and Review of the Literature
Journal of Fungi Review Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature Akaninyene Otu 1,2,*, Philip Langridge 2 and David W. Denning 2,3 1 Department of Internal Medicine, College of Medical Sciences, University of Calabar, Calabar, Cross River State P.M.B. 1115, Nigeria 2 The National Aspergillosis Centre, 2nd Floor Education and Research Centre, Wythenshawe Hospital, Southmoor Road, Manchester M23 9LT, UK; [email protected] (P.L.); [email protected] (D.W.D.) 3 Faculty of Biology, Medicine and Health, The University of Manchester, and Manchester Academic Health Science Centre, Oxford Rd, Manchester M13 9PL, UK * Correspondence: [email protected] Received: 5 September 2018; Accepted: 8 October 2018; Published: 15 October 2018 Abstract: Many chronic lung diseases are characterized by the hypersecretion of mucus. In these conditions, the administration of mucoactive agents is often indicated as adjuvant therapy. N-acetylcysteine (NAC) is a typical example of a mucolytic agent. A retrospective review of patients with pulmonary aspergillosis treated at the National Aspergillosis Centre in Manchester, United Kingdom, with NAC between November 2015 and November 2017 was carried out. Six Caucasians with Aspergillus lung disease received NAC to facilitate clearance of their viscid bronchial mucus secretions. One patient developed immediate bronchospasm on the first dose and could not be treated. Of the remainder, two (33%) derived benefit, with increased expectoration and reduced symptoms. Continued response was sustained over 6–7 months, without any apparent toxicity. In addition, a systematic review of the literature is provided to analyze the utility of NAC in the management of respiratory conditions which have unresponsive bronchial obstruction as a feature. -

Blue Cross and Blue Shield January 2018 5 Tier Basic Drug List
January 2018 5 Tier Basic Drug List Please consider talking to your doctor about prescribing preferred medications, which may help reduce your out-of-pocket costs. This list may help guide you and your doctor in selecting an appropriate medication for you. The drug list is regularly updated. You can view the most up-to-date list, or the specialty drug list, at MyPrime.com. Contents Therapeutic Class Drug List Introduction ....................................................... I Anti-Infective Agents ........................................ 1 How drugs are selected .................................... I Cancer Drugs ................................................... 3 How member payment is determined ............... I Hormones, Diabetes and Related Drugs ......... 5 How to use this list ............................................ I Heart and Circulatory Drugs ............................ 9 Generic drugs .................................................. II Respiratory Agents ........................................ 14 Coverage considerations ................................ III Gastrointestinal Drugs ................................... 16 Specialty drugs .............................................. IV Genitourinary Drugs ....................................... 17 Central Nervous System Drugs ..................... 1 Abbreviation/acronym key .............................. V 8 Pain Relief Drugs ........................................... 21 Neuromuscular Drugs .................................... 23 Supplements .................................................