International Nonproprietary Names for Pharmaceutical Substances (INN)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -
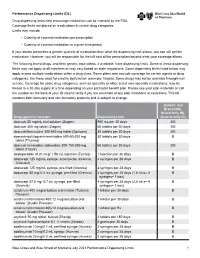
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -
![Full Prescribing Information [FDA]](https://docslib.b-cdn.net/cover/1742/full-prescribing-information-fda-701742.webp)
Full Prescribing Information [FDA]
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------------CONTRAINDICATIONS------------------------------ These highlights do not include all the information needed to use None. MONJUVI safely and effectively. See full prescribing information for MONJUVI. ------------------------WARNINGS AND PRECAUTIONS----------------------- Infusion-Related Reactions: Monitor patients frequently during infusion. MONJUVI® (tafasitamab-cxix) for injection, for intravenous use Interrupt or discontinue infusion based on severity. (2.3, 5.1) Initial U.S. Approval: 2020 Myelosuppression: Monitor complete blood counts. Manage using dose modifications and growth factor support. Interrupt or discontinue -----------------------------INDICATIONS AND USAGE-------------------------- MONJUVI based on severity. (2.3, 5.2) MONJUVI is a CD19-directed cytolytic antibody indicated in combination Infections: Bacterial, fungal and viral infections can occur during and with lenalidomide for the treatment of adult patients with relapsed or following MONJUVI. Monitor patients for infections. (2.3, 5.3) refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, Embryo-Fetal Toxicity: May cause fetal harm. Advise females of including DLBCL arising from low grade lymphoma, and who are not eligible reproductive potential of the potential risk to a fetus and use of effective for autologous stem cell transplant (ASCT). contraception. (5.4) This indication is approved under accelerated approval based on overall -------------------------------ADVERSE -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

Rxoutlook® 1St Quarter 2019
® RxOutlook 1st Quarter 2020 optum.com/optumrx a RxOutlook 1st Quarter 2020 Orphan drugs continue to feature prominently in the drug development pipeline In 1983 the Orphan Drug Act was signed into law. Thirty seven years later, what was initially envisioned as a minor category of drugs has become a major part of the drug development pipeline. The Orphan Drug Act was passed by the United States Congress in 1983 in order to spur drug development for rare conditions with high unmet need. The legislation provided financial incentives to manufacturers if they could demonstrate that the target population for their drug consisted of fewer than 200,000 persons in the United States, or that there was no reasonable expectation that commercial sales would be sufficient to recoup the developmental costs associated with the drug. These “Orphan Drug” approvals have become increasingly common over the last two decades. In 2000, two of the 27 (7%) new drugs approved by the FDA had Orphan Designation, whereas in 2019, 20 of the 48 new drugs (42%) approved by the FDA had Orphan Designation. Since the passage of the Orphan Drug Act, 37 years ago, additional regulations and FDA designations have been implemented in an attempt to further expedite drug development for certain serious and life threatening conditions. Drugs with a Fast Track designation can use Phase 2 clinical trials to support FDA approval. Drugs with Breakthrough Therapy designation can use alternative clinical trial designs instead of the traditional randomized, double-blind, placebo-controlled trial. Additionally, drugs may be approved via the Accelerated Approval pathway using surrogate endpoints in clinical trials rather than clinical outcomes. -

Corporate Medical Policy Tafasitamab-Cxix (Monjuvi®)
Corporate Medical Policy Tafasitamab-cxix (Monjuvi®) File Name: tafasitamab_monjuvi Origination: 10/2020 Last CAP Review: 1/2021 Next CAP Review: 1/2022 Last Review: 1/2021 Description of Procedure or Service Tafasitamab-cxix (Monjuvi®) is a CD19-directed cytolytic antibody indicated in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT). DLBCL is the most common subtype of non-Hodgkin lymphoma (NHL), representing approximately one-third of NHL patients. It is an aggressive and quickly progressing NHL in which survival without treatment is measured in months. While there have been many advancements in the treatment of DLBCL, a cure is not achieved in most patients using conventional therapy, and roughly 30 to 40% of patients suffer relapse. For patients who are refractory to initial treatment and for patients who relapse after an initial response, only a small percentage will experience prolonged disease-free survival with salvage chemoimmunotherapy treatment alone. The preferred treatment for a first relapse of DLBCL or primary refractory DLBCL is salvage chemoimmunotherapy followed by autologous hematopoietic stem cell transplantation (HSCT). However, the outcome for relapsed/refractory DLBCL patients who are ineligible for transplant remains poor. Tafasitamab-cxix (Monjuvi) is a humanized CD19-directed cytolytic monoclonal antibody, which was approved by the U.S. Food and Drug Administration (FDA) in July 2020 for the treatment of relapsed or refractory DLBCL. It works by binding to CD19 expressed on the surface of pre-B and mature B lymphocytes and on several B-cell malignancies, including DLBCL, which induces B-cell lysis via apoptosis and immune effector mechanisms. -

Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary
Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary Effective September 1, 2021 Health Plan Products: Kaiser Permanente Bernard J. Tyson School of Medicine, EPO Student Blanket Health Plan offered by Kaiser Permanente Insurance Company For the most current list of covered medications or for help understanding your KPIC insurance plan benefits, including cost sharing for drugs under the prescription drug benefit and under the medical benefit: Call 1-800-533-1833, TTY 711, Monday through Friday, 7 a.m. to 9 p.m. ET Visit kaiserpermanente.org to: • Find a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. • Find an electronic copy of the formulary here. • Get plan coverage information. For cost sharing information for the outpatient prescription drug benefits in your specific plan, please visit kp.org/kpic-websiteTBD The formulary is subject to change and all previous versions of the formulary are no longer in effect. Kaiser Permanente Last updated: September 1, 2021 Table of Contents Informational Section...........................................................................................................................................3 ANTIHISTAMINE DRUGS - Drugs for Allergy.....................................................................................................9 ANTI-INFECTIVE AGENTS - Drugs for Infections........................................................................................... -

Antibodies for the Treatment of Brain Metastases, a Dream Or a Reality?
pharmaceutics Review Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Marco Cavaco, Diana Gaspar, Miguel ARB Castanho * and Vera Neves * Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal * Correspondence: [email protected] (M.A.R.B.C.); [email protected] (V.N.) Received: 19 November 2019; Accepted: 28 December 2019; Published: 13 January 2020 Abstract: The incidence of brain metastases (BM) in cancer patients is increasing. After diagnosis, overall survival (OS) is poor, elicited by the lack of an effective treatment. Monoclonal antibody (mAb)-based therapy has achieved remarkable success in treating both hematologic and non-central-nervous system (CNS) tumors due to their inherent targeting specificity. However, the use of mAbs in the treatment of CNS tumors is restricted by the blood–brain barrier (BBB) that hinders the delivery of either small-molecules drugs (sMDs) or therapeutic proteins (TPs). To overcome this limitation, active research is focused on the development of strategies to deliver TPs and increase their concentration in the brain. Yet, their molecular weight and hydrophilic nature turn this task into a challenge. The use of BBB peptide shuttles is an elegant strategy. They explore either receptor-mediated transcytosis (RMT) or adsorptive-mediated transcytosis (AMT) to cross the BBB. The latter is preferable since it avoids enzymatic degradation, receptor saturation, and competition with natural receptor substrates, which reduces adverse events. Therefore, the combination of mAbs properties (e.g., selectivity and long half-life) with BBB peptide shuttles (e.g., BBB translocation and delivery into the brain) turns the therapeutic conjugate in a valid approach to safely overcome the BBB and efficiently eliminate metastatic brain cells. -

Treating Non-Hodgkin Lymphoma If You’Ve Been Diagnosed with Non-Hodgkin Lymphoma, Your Treatment Team Will Discuss Your Options with You
cancer.org | 1.800.227.2345 Treating Non-Hodgkin Lymphoma If you’ve been diagnosed with non-Hodgkin lymphoma, your treatment team will discuss your options with you. It’s important to weigh the benefits of each treatment option against the possible risks and side effects. How is non-Hodgkin lymphoma treated? Depending on the type and stage (extent) of the lymphoma and other factors, treatment options for people with NHL might include: ● Chemotherapy for Non-Hodgkin Lymphoma ● Immunotherapy for Non-Hodgkin Lymphoma ● Targeted Drug Therapy for Non-Hodgkin Lymphoma ● Radiation Therapy for Non-Hodgkin Lymphoma ● High-Dose Chemotherapy and Stem Cell Transplant for Non-Hodgkin Lymphoma ● Surgery for Non-Hodgkin Lymphoma Common treatment approaches Treatment approaches for NHL depend on the type of cancer, how advanced it is, as well as your health and other factors. Another important part of treatment for many people is palliative or supportive care. This can help prevent or treat problems such as infections, low blood cell counts, or some symptoms caused by the lymphoma. ● Treating B-Cell Non-Hodgkin Lymphoma ● Treating T-Cell Non-Hodgkin Lymphoma ● Treating HIV-Associated Lymphoma 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● Palliative and Supportive Care for Non-Hodgkin Lymphoma Who treats non-Hodgkin lymphoma? Based on your treatment options, you may have different types of doctors on your treatment team. These doctors could include: ● A medical oncologist or hematologist: a doctor who treats lymphoma with chemotherapy, immunotherapy, and targeted therapy. ● A radiation oncologist: a doctor who treats cancer with radiation therapy. ● A bone marrow transplant doctor: a doctor who specializes in treating cancer or other diseases with bone marrow or stem cell transplants. -

Current Immunotherapy Approaches in Non-Hodgkin Lymphomas
Review Current Immunotherapy Approaches in Non-Hodgkin Lymphomas Robert Pytlik 1,2, Kamila Polgarova 2, Jana Karolova 2,3 and Pavel Klener 2,3,* 1 Institute of Haematology and Blood Transfusion, 128 00 Prague, Czech Republic; [email protected] 2 First Department of Internal Medicine-Hematology, University General Hospital in Prague and First Faculty of Medicine, Charles University, U Nemocnice 2, 128 08 Prague 2, 110 00 Prague, Czech Republic; [email protected] (K.P.); [email protected] (J.K.) 3 Institute of Pathological Physiology, First Faculty of Medicine, Charles University, 128 53 Prague, Czech Republic * Correspondence: [email protected]; Tel.: +420-224965933 Received: 30 October 2020; Accepted: 24 November 2020; Published: 27 November 2020 Abstract: Non-Hodgkin lymphomas (NHLs) are lymphoid malignancies of B- or T-cell origin. Despite great advances in treatment options and significant improvement of survival parameters, a large part of NHL patients either present with a chemotherapy-refractory disease or experience lymphoma relapse. Chemotherapy-based salvage therapy of relapsed/refractory NHL is, however, capable of re-inducing long-term remissions only in a minority of patients. Immunotherapy-based approaches, including bispecific antibodies, immune checkpoint inhibitors and genetically engineered T-cells carrying chimeric antigen receptors, single-agent or in combination with therapeutic monoclonal antibodies, immunomodulatory agents, chemotherapy or targeted agents demonstrated unprecedented clinical activity in heavily-pretreated patients with NHL, including chemotherapy-refractory cases with complex karyotype changes and other adverse prognostic factors. In this review, we recapitulate currently used immunotherapy modalities in NHL and discuss future perspectives of combinatorial immunotherapy strategies, including patient-tailored approaches. -

Unitedhealthcare Cancer Therapy Pathways Program Regimens
UnitedHealthcare Cancer Therapy Pathways Program Overview 2 Breast Cancer 3 Pancreatic Cancer 15 Melanoma 19 Colon/Rectal Cancer 23 Hepatobiliary Cancers 30 Lung Cancer, Small Cell 35 Lung Cancer, Non-Small Cell 40 Ovarian, Fallopian and Primary Peritoneal Cancer 56 Head and Neck Cancer 67 Multiple Myeloma 74 Lymphoma, Diffuse Large B-Cell 86 Lymphoma, Follicular 92 Lymphoma, Marginal Zone 97 Lymphoma, Mantle Cell 101 Change control 1 OVERVIEW Cancer Therapy Pathways Program With the rapid approval of new therapies, along with the rising cost of cancer care, pathways serve a critical role in the delivery of high-quality and high-value cancer treatments while reducing an unwarranted variation in care. The UnitedHealthcare Cancer Therapy Pathways Program aims to improve quality and value in cancer care by identifying anti-cancer regimens supported by evidence-based guidelines to help reduce total cost of care and improve outcomes. The program’s regimens are selected on the basis of clinical benefit (efficacy) and side-effect profile (toxicity). Among regimens with comparable efficacy and toxicity, additional consideration is given to the frequency of hospitalizations during therapy, duration of therapy, drug costs and total cost of care. Care decisions are between the physician and the patient The Cancer Therapy Pathways Program is not a substitute for the experience and judgment of a physician or other health care professional. Any clinician participating in the program must use independent medical judgment in the context of individual -

Tafasitamab-Cxix)
Drug Policy: Monjuvi™ (tafasitamab-cxix) POLICY NUMBER SUBJECT DEPT/PROGRAM PAGE 1 OF 3 UM ONC_1412 Monjuvi™ (tafasitamab-cxix) UM Dept DATES COMMITTEE REVIEWED APPROVAL DATE EFFECTIVE DATE COMMITTEE APPROVAL DATES 09/09/20 September 9, 2020 September 25, 2020 (latest version listed last) 09/09/20 PRIMARY BUSINESS OWNER: UM COMMITTEE/BOARD APPROVAL APPROVED BY: Dr. Andrew Hertler Utilization Management Committee URAC STANDARDS NCQA STANDARDS ADDITIONAL AREAS OF IMPACT HUM 1 UM 2 CMS REQUIREMENTS STATE/FEDERAL REQUIREMENTS APPLICABLE LINES OF BUSINESS Commercial, Exchange, Medicaid I. PURPOSE To define and describe the accepted indications for Monjuvi (tafasitamab-cxix) usage in the treatment of cancer, including FDA approved indications, and off-label indications. New Century Health (NCH) is responsible for processing all medication requests from network ordering providers. Medications not authorized by NCH may be deemed as not approvable and therefore not reimbursable. The use of this drug must be supported by one of the following: FDA approved product labeling, CMS-approved compendia, National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO) clinical guidelines, or peer-reviewed literature that meets the requirements of the CMS Medicare Benefit Policy Manual Chapter 15. II. INDICATIONS FOR USE/INCLUSION CRITERIA A. PREFERRED MEDICATION GUIDANCE FOR INITIAL REQUEST: 1. When health plan Medicaid coverage provisions—including any applicable PDLs (Preferred Drug Lists)—conflict with the coverage provisions in this drug policy, health plan Medicaid coverage provisions take precedence per the Preferred Drug Guidelines OR 2. When health plan Exchange coverage provisions-including any applicable PDLs (Preferred Drug Lists)-conflict with the coverage provisions in this drug policy, health plan Exchange coverage provisions take precedence per the Preferred Drug Guidelines OR 3.