Enhanced Annual Drug List Dispensing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Maintenance Drug List
Commonly Prescribed Maintenance Medications Maintenance (or long-term) medications are those dosage form may be used to treat more than one drugs you may take on a regular basis to treat conditions medical condition. In these cases, each medication may such as high cholesterol, high blood pressure or diabetes. be classified according to its first U.S. Food and Drug Based on your benefit plan, you may get up to a 90-day Administration (FDA) approved use. Some strengths supply of covered maintenance medication delivered and/or formulations listed may not be covered. to you through a home delivery program or at select Please note that oral contraceptives are maintenance retail pharmacies. medications and are part of this list. However, this Some plans may require you to fill these prescriptions list does not include the trade names of all available through home delivery or at select retail pharmacies oral contraceptives because there are a large number in order to receive coverage. of products. Commonly used maintenance medications are listed in Coverage is always subject to the terms and limits of this guide. This list is not all-inclusive and may change your benefit plan. Please verify with your plan if there from time to time. are any additional requirements before a drug may be Most generic drugs listed are followed by a reference covered. For details about your plan, check your benefit brand drug in (parentheses). The brand name drug materials or call the number on your member ID card. in parentheses is listed for reference and may not be Third-party brand names are the property of their covered under your benefit. -

VITAL Jan 2019 Follow-Up
VITAL 6308 Request Use ball-point pen to complete the form. OBS 1 DATE OF BIRTH: / / We use DATE OF BIRTH (DOB) to verify the identity of the person providing information. Is the DOB above correct? Yes No IF NO, what is your correct date of birth? / / s. Peripheral artery surgery / 1. IN THE PAST YEAR, have you been NEWLY DIAGNOSED stenting (procedure to No Yes / with any of the following? IF YES, please provide the unblock arteries in legs) month/year of the NEW diagnosis or procedure. t. Carotid stenosis (blocked No Yes Answer NO/YES on each line. arteries in neck) / Diagnosis (Please complete either N/Y for each item) MO/YR u. Carotid artery surgery / stenting (procedure to No Yes / a. Hypertension (high blood pressure) No Yes / unblock arteries in neck) v. Deep vein thrombosis No Yes (blood clot in legs) / b. Diabetes No Yes / w. Pulmonary embolism No Yes (blood clot in lungs) / c. Cancer (NOT including skin cancer) No Yes / IF YES, specify type: _______________________ x. Parkinson's disease No Yes / d. Skin cancer No Yes / y. Multiple sclerosis No Yes IF YES, specify type: / e. melanoma squamous or basal cell not sure z. Cataract surgery (extraction) No Yes / f. Heart attack or myocardial infarction No Yes / aa. Macular degeneration No Yes / g. Coronary bypass surgery No Yes / bb. Dry eye syndrome No Yes or dry eye disease / h. Coronary angioplasty or stent No Yes (balloon used to unblock an artery) / cc. Periodontal disease No Yes (gum disease) / i. Chest pain (angina) No Yes / IF YES, were you hospitalized? No Yes dd. -

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -
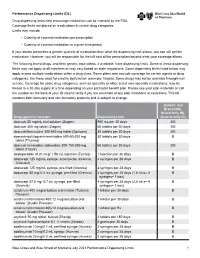
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Lifitegrast for the Treatment of Dry Eye Disease in Adults
Expert Opinion on Pharmacotherapy ISSN: 1465-6566 (Print) 1744-7666 (Online) Journal homepage: http://www.tandfonline.com/loi/ieop20 Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg To cite this article: Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg (2017): Lifitegrast for the treatment of dry eye disease in adults, Expert Opinion on Pharmacotherapy, DOI: 10.1080/14656566.2017.1372748 To link to this article: http://dx.doi.org/10.1080/14656566.2017.1372748 Accepted author version posted online: 25 Aug 2017. Published online: 04 Sep 2017. Submit your article to this journal Article views: 11 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ieop20 Download by: [108.6.184.233] Date: 04 September 2017, At: 04:29 EXPERT OPINION ON PHARMACOTHERAPY, 2017 https://doi.org/10.1080/14656566.2017.1372748 DRUG EVALUATION Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfelda, Henry D. Perryb, Alanna S. Nattisb and Eric D. Rosenbergc aOphthalmic Consultants of Long Island, New York University Medical Center, Garden City, NY, USA; bOphthalmic Consultants of Long Island, Nassau University Medical Center, Rockville Centre, NY, USA; cWestchester Medical Center, Valhalla, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Dry eye disease (DED) is a common ocular disorder that can have a substantial burden on Received 14 June 2017 quality of life and daily activities. Lifitegrast ophthalmic solution 5.0% is the first medication approved in Accepted 24 August 2017 the US for the treatment of the signs and symptoms of DED. -

S41598-020-80397-9.Pdf
www.nature.com/scientificreports OPEN Activation of PKC supports the anticancer activity of tigilanol tiglate and related epoxytiglianes Jason K. Cullen1, Glen M. Boyle1,2,3,7*, Pei‑Yi Yap1, Stefan Elmlinger1, Jacinta L. Simmons1, Natasa Broit1, Jenny Johns1, Blake Ferguson1, Lidia A. Maslovskaya1,4, Andrei I. Savchenko4, Paul Malek Mirzayans4, Achim Porzelle4, Paul V. Bernhardt4, Victoria A. Gordon5, Paul W. Reddell5, Alberto Pagani6, Giovanni Appendino6, Peter G. Parsons1,3 & Craig M. Williams4* The long‑standing perception of Protein Kinase C (PKC) as a family of oncoproteins has increasingly been challenged by evidence that some PKC isoforms may act as tumor suppressors. To explore the hypothesis that activation, rather than inhibition, of these isoforms is critical for anticancer activity, we isolated and characterized a family of 16 novel phorboids closely‑related to tigilanol tiglate (EBC‑46), a PKC‑activating epoxytigliane showing promising clinical safety and efcacy for intratumoral treatment of cancers. While alkyl branching features of the C12‑ester infuenced potency, the 6,7‑epoxide structural motif and position was critical to PKC activation in vitro. A subset of the 6,7‑epoxytiglianes were efcacious against established tumors in mice; which generally correlated with in vitro activation of PKC. Importantly, epoxytiglianes without evidence of PKC activation showed limited antitumor efcacy. Taken together, these fndings provide a strong rationale to reassess the role of PKC isoforms in cancer, and suggest in some situations their activation can be a promising strategy for anticancer drug discovery. Te Protein Kinase C (PKC) family of serine/threonine kinases was frst identifed almost 40 years ago1. -

MSM Chapter 1200 3/1/21
MEDICAID SERVICES MANUAL TRANSMITTAL LETTER February 23, 2021 TO: CUSTODIANS OF MEDICAID SERVICES MANUAL FROM: JESSICA KEMMERER, HIPAA PRIVACY AND CIVIL RIGHTS OFFICER /Jessica Kemmerer/ BACKGROUND AND EXPLANATION The DHCFP is proposing revisions to Medicaid Services Manual (MSM), Chapter 1200 – Prescribed Drugs, Appendix A, to reflect recommendations approved on October 22, 2020, by the Drug Use Review (DUR) Board. The proposed changes include the addition of new prior authorization criteria for Doxepine Topical, the addition of new prior authorization criteria for Zeposia® (ozanimod), addition of new prior authorization for Evenity® (romosozumab-aqqg), Prolia® (denosumab), Forteo® (teriparatide) and Tymlos® (abaloparatide) within a new combined osteoporosis agents section, and addition of new prior authorization criteria for Orilissa® (elagolix) and Oriahnn® (elagolix, estradiol, and norethindrone) within a new Gonadorpin Hormone Receptor (GnRH) Antagonist and Combinations section. Additionally, the DHCFP is proposing revisions to the existing prior authorization criteria for psychotropic medications for children and adolescents, and revision to the existing clinical criteria for Epidiolex® (cannabidiol). Throughout the chapter, grammar, punctuation and capitalization changes were made, duplications removed, acronyms used and standardized, and language reworded for clarity. Renumbering and re- arranging of sections was necessary. These changes are effective March 1, 2021. MATERIAL TRANSMITTED MATERIAL SUPERSEDED MTL N/A MTL N/A MSM Ch 1200 – Prescribed Drugs MSM Ch 1200 – Prescribed Drugs Background and Explanation of Policy Changes, Manual Section Section Title Clarifications and Updates Appendix A Psychotropic Added new policy language criteria on which specific Section N Medications for drug classes may bypass polypharmacy clinical criteria. Children and Adolescents Appendix A Reserved for Future Created a new section titled “Doxepin Topical.” Added Section W Use new prior authorization criteria for doxepin topical. -

The Effect of Wool Hydrolysates on Squamous Cell Carcinoma Cells in Vitro
RESEARCH ARTICLE The effect of wool hydrolysates on squamous cell carcinoma cells in vitro. Possible implications for cancer treatment Tatsiana Damps1*, Anna Katarzyna Laskowska1, Tomasz Kowalkowski2,3, Monika Prokopowicz4, Anna Katarzyna Puszko5, Piotr Sosnowski1, Joanna Czuwara6, Marek Konop6, Krzysztof RoÂżycki4, Joanna Karolina Borkowska7, Aleksandra Misicka1, Lidia Rudnicka1 1 Department of Neuropeptides, Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland, 2 Chair of Environmental Chemistry and Bioanalytics, Faculty of Chemistry, Nicolaus a1111111111 Copernicus University, Toruń, Poland, 3 Interdisciplinary Centre of Modern Technology, Nicolaus Copernicus a1111111111 University, Toruń, Poland, 4 Laboratory of Chemical Synthesis, CePT, Mossakowski Medical Research a1111111111 Centre, Polish Academy of Sciences, Warsaw, Poland, 5 Faculty of Chemistry, University of Warsaw, a1111111111 Warsaw, Poland, 6 Department of Dermatology, Medical University of Warsaw, Warsaw, Poland, 7 Department of Human Epigenetics, Mossakowski Medical Research Centre, Polish Academy of Sciences, a1111111111 Warsaw, Poland * [email protected] OPEN ACCESS Abstract Citation: Damps T, Laskowska AK, Kowalkowski T, Prokopowicz M, Puszko AK, Sosnowski P, et al. Squamous cell carcinoma of the skin is the second most common cutaneous malignancy. (2017) The effect of wool hydrolysates on Despite various available treatment methods and advances in noninvasive diagnostic tech- squamous cell carcinoma cells in vitro. Possible niques, the incidence of metastatic cutaneous squamous cell carcinoma is rising. Deficiency implications for cancer treatment. PLoS ONE 12 (8): e0184034. https://doi.org/10.1371/journal. in effective preventive or treatment methods of transformed keratinocytes leads to necessity pone.0184034 of searching for new anticancer agents. The present study aims to evaluate the possibility of Editor: Andrzej T Slominski, University of Alabama using wool hydrolysates as such agents. -

Summary of Appeals & Independent Review Organization
All Other Appeals All other appeals are for drugs not in an inpatient hospital setting that Molina was not able to approve. Sometimes, the clinical information sent to us for these drugs do not meet medical necessity on initial review. When drug preauthorization requests are denied, a member or provider has the right to appeal. Appeals allow time to provide more clinical information. With complete clinical information, we can usually approve the drug. These are considered an appeal overturn. When the denial decision is not overturned, it is considered upheld. Service Code/Drug Name Service Code Description Number of Appeals Number of Appeals Total Appeals Upheld Overturned A9274 EXTERNAL AMB INSULIN DEL SYSTEM DISPOSABLE EA 0 1 1 Abatacept 3 1 4 Abemaciclib 1 0 1 Acalabrutinib 0 1 1 Acne Combination - Two Ingredient 1 0 1 Acyclovir 0 1 1 Adalimumab 7 14 21 Adrenergic Combination - Two Ingredient 1 0 1 Aflibercept 0 2 2 Agalsidase 1 0 1 Alfuzosin 1 0 1 Amantadine 1 0 1 Ambrisentan 0 1 1 Amphetamine 0 1 1 Amphetamine Mixtures - Two Ingredient 1 8 9 Apixaban 7 21 28 Apremilast 12 13 25 Aprepitant 0 1 1 Aripiprazole 5 9 14 ARNI-Angiotensin II Recept Antag Comb - Two Ingredient 6 9 15 Asenapine 0 1 1 Atomoxetine 1 3 4 Atorvastatin 0 1 1 Atovaquone 1 0 1 Axitinib 0 1 1 Azathioprine 0 1 1 Azilsartan 1 0 1 Azithromycin 1 0 1 Baclofen 0 1 1 Baricitinib 1 1 2 Belimumab 0 1 1 Benralizumab 1 0 1 Beta-blockers - Ophthalmic Combination - Two Ingredient 0 2 2 Bimatoprost 0 1 1 Botulinum Toxin 1 4 5 Buprenorphine 4 3 7 Calcifediol 1 0 1 Calcipotriene -

2017 Fda Peptide Harvest
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 April 2018 doi:10.20944/preprints201804.0126.v1 Peer-reviewed version available at Pharmaceuticals 2018, 11, 42; doi:10.3390/ph11020042 1 Review 2 2017 FDA PEPTIDE HARVEST 3 Othman Al Musaimi,1,2,# Danah Alshaer, 1,2,# Beatriz G. de la Torre,3,* Fernando Albericio,2,4,5.* 4 1 College of Health Sciences, University of KwaZulu-Natal, Durban 4000, South Africa 5 2 School of Chemistry, University of KwaZulu-Natal, Durban 4001, South Africa 6 3 KRISP, College of Health Sciences, University of KwaZulu-Natal, Durban 4001, South Africa 7 4 CIBER-BBN, Networking Centre on Bioengineering, Biomaterials and Nanomedicine, University of 8 Barcelona, 08028 Barcelona, Spain 9 5 Department of Organic Chemistry, University of Barcelona, 08028 Barcelona, Spain 10 * Correspondence: [email protected]; [email protected]; Tel.: +27-614009144 11 12 13 Abstract: 2017 was an excellent year in terms of new drugs (chemical entities and biologics) 14 approved by the FDA, with a total of forty-six. In turn, one of the highlights was the number of 15 peptides (six) included in this list. Here, the six peptides are analysed in terms of chemical structure, 16 synthetic strategy used for their production, source, biological target, and mode of action. 17 Keywords: pharmaceutical market; drugs; drug discovery; solid-phase peptide synthesis 18 Introduction 19 The financial investment associated with the pharmaceutical industry is one of the largest in the 20 industrial sector—surpassed only by the telecommunications sector. However, the number of new 21 products (drugs) entering the market each year is relatively low.