ATC Vet 2021 Atccode Atcdescription Atcdescriptionsi QA ALIMENTARY TRACT and METABOLISM ZDRAVILA ZA BOLEZNI PREBAVIL in PRESNOVE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ontario Drug Benefit Formulary Edition 43
Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/Comparative Drug Index Edition 43 Drug Programs Policy and Strategy Branch Ontario Public Drug Programs Ministry of Health and Long-Term Care Effective February 28, 2018 Visit Formulary Downloads: Edition 43 Table of Contents Part I Introduction ....................................................................................................... I.1 Part II Preamble .......................................................................................................... II.1 Part III-A Benefits List ........................................................................................... III-A.1 Part III-B Off-Formulary Interchangeable Drugs (OFI) ........................................ III-B.1 Part IV Section Currently Not In Use ......................................................................... IV Part V Index of Pharmacologic-Therapeutic Classification .................................... V.1 Part VI-A Facilitated Access - HIV/AIDS .............................................................. VI-A.1 Part VI-B Facilitated Access - Palliative Care ..................................................... VI-B.1 Part VI-C Temporary Facilitated Access - Rheumatology ................................. VI-C.1 Part VII Trillium Drug Program ................................................................................ VII.1 Part VIII Exceptional Access Program (EAP) ........................................................ VIII.1 Part IX-A Nutrition Products ................................................................................ -

Lipid Lowering Drugs and Inflammatory Changes: an Impact on Cardiovascular Outcomes?
Annals of Medicine ISSN: 0785-3890 (Print) 1365-2060 (Online) Journal homepage: http://www.tandfonline.com/loi/iann20 Lipid Lowering Drugs and Inflammatory Changes: an Impact on Cardiovascular Outcomes? M. Ruscica, N. Ferri, C. Macchi, A. Corsini & C. R. Sirtori To cite this article: M. Ruscica, N. Ferri, C. Macchi, A. Corsini & C. R. Sirtori (2018): Lipid Lowering Drugs and Inflammatory Changes: an Impact on Cardiovascular Outcomes?, Annals of Medicine, DOI: 10.1080/07853890.2018.1498118 To link to this article: https://doi.org/10.1080/07853890.2018.1498118 Accepted author version posted online: 06 Jul 2018. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=iann20 LIPID LOWERING DRUGS AND INFLAMMATORY CHANGES: AN IMPACT ON CARDIOVASCULAR OUTCOMES? M. Ruscica1*, N. Ferri2*, C. Macchi1, A. Corsini1 and C. R. Sirtori3 1Dipartimento di Scienze Farmacologiche e Biomolecolari, Università degli Studi di Milano, Milan, Italy; 2Dipartimento di Scienze del Farmaco, Università degli Studi di Padova, Padova, Italy; 3Centro Dislipidemie, A.S.S.T. Grande Ospedale Metropolitano Niguarda, Milan, Italy *Both authors contributed equally to this work Corresponding Author: Cesare R. Sirtori [email protected] Abstract Inflammatory changes are responsible for maintenance of the atherosclerotic process and may underlie some of the most feared vascular complications. Among the multiple mechanisms of inflammation, the arterial deposition of lipids and particularly of cholesterol crystals is the one responsible for activation of inflammasome NLRP3, followed by the rise of circulating markers, mainly C-reactive protein (CRP). Elevation of lipoproteins, LDL but also VLDL and remnants, associates with increased inflammatory changes and coronary risk. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease
nutrients Review Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease Cong Xie 1,† , Weikun Huang 1,2,† , Richard L. Young 1,3 , Karen L. Jones 1,4 , Michael Horowitz 1,4, Christopher K. Rayner 1,5 and Tongzhi Wu 1,4,6,* 1 Adelaide Medical School, Center of Research Excellence (CRE) in Translating Nutritional Science to Good Health, The University of Adelaide, Adelaide 5005, Australia; [email protected] (C.X.); [email protected] (W.H.); [email protected] (R.L.Y.); [email protected] (K.L.J.); [email protected] (M.H.); [email protected] (C.K.R.) 2 The ARC Center of Excellence for Nanoscale BioPhotonics, Institute for Photonics and Advanced Sensing, School of Physical Sciences, The University of Adelaide, Adelaide 5005, Australia 3 Nutrition, Diabetes & Gut Health, Lifelong Health Theme South Australian Health & Medical Research Institute, Adelaide 5005, Australia 4 Endocrine and Metabolic Unit, Royal Adelaide Hospital, Adelaide 5005, Australia 5 Department of Gastroenterology and Hepatology, Royal Adelaide Hospital, Adelaide 5005, Australia 6 Institute of Diabetes, School of Medicine, Southeast University, Nanjing 210009, China * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Bile acids are cholesterol-derived metabolites with a well-established role in the digestion and absorption of dietary fat. More recently, the discovery of bile acids as natural ligands for the nuclear farnesoid X receptor (FXR) and membrane Takeda G-protein-coupled receptor 5 (TGR5), and Citation: Xie, C.; Huang, W.; Young, the recognition of the effects of FXR and TGR5 signaling have led to a paradigm shift in knowledge R.L.; Jones, K.L.; Horowitz, M.; regarding bile acid physiology and metabolic health. -

Changes to the Highmark Drug Formularies
AUGUST 2020 JULY/AUGUST 2020 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for July/August 2020. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in June 2020 by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Progressive Formulary and the Highmark Progressive Healthcare Reform Formulary C. Changes to the Highmark Healthcare Reform Essential Formulary D. Changes to the Highmark Core Formulary E. Changes to the Highmark National Select Formulary F. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs Section II. Highmark Medicare Part D Formularies A. Changes to the Highmark Medicare Part D 5-Tier Incentive Formulary B. Changes to the Highmark Medicare Part D 5-Tier Closed Formulary C. Additions to the Specialty Tier D. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Quantity Level Limit (QLL) Program As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. -

BLA 761125 Page 7
BLA 761125 Page 7 HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use BEOVU Endophthalmitis and retinal detachments may occur following intravitreal safely and effectively. See full prescribing information for BEOVU. injections. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay (5.1). BEOVU® (brolucizumab-dbll) injection, for intravitreal injection Increases in intraocular pressure (IOP) have been seen within 30 minutes of Initial U.S. Approval: 2019 an intravitreal injection (5.2). ----------------------------INDICATIONS AND USAGE------------------------- There is a potential risk of arterial thromboembolic events (ATE) following BEOVU is a human vascular endothelial growth factor (VEGF) inhibitor intravitreal use of VEGF inhibitors (5.3). indicated for the treatment of Neovascular (Wet) Age-Related Macular ------------------------------ADVERSE REACTIONS----------------------------- Degeneration (AMD) (1). The most common adverse reactions (≥ 5%) reported in patients receiving ----------------------DOSAGE AND ADMINISTRATION---------------------- BEOVU are vision blurred (10%), cataract (7%), conjunctival hemorrhage BEOVU is administered by intravitreal injection. The recommended dose for (6%), eye pain (5%), and vitreous floaters (5%) (6.1). BEOVU is 6 mg (0.05 mL of 120 mg/mL solution) monthly (approximately To report SUSPECTED ADVERSE REACTIONS, contact Novartis every 25-31 days) for the first three doses, followed by one dose of 6 mg (0.05 Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA mL) every 8-12 weeks (2). 1088 or www.fda.gov/medwatch. ---------------------DOSAGE FORMS AND STRENGTHS-------------------- See 17 for PATIENT COUNSELING INFORMATION. Injection: 6 mg/0.05 mL solution for intravitreal injection in a single-dose vial (3). -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Abstract Book
ISSN 0390-6078 Volume 105 OCTOBER 2020 - S2 XVI Congress of the Italian Society of Experimental Hematology Napoli, Italy, October 15-17, 2020 ABSTRACT BOOK www.haematologica.org XVI Congress of the Italian Society of Experimental Hematology Napoli, Italy, October 15-17, 2020 COMITATO SCIENTIFICO Pellegrino Musto, Presidente Antonio Curti, Vice Presidente Mario Luppi, Past President Francesco Albano Niccolò Bolli Antonella Caivano Roberta La Starza Luca Malcovati Luca Maurillo Stefano Sacchi SEGRETERIA SIES Via De' Poeti, 1/7 - 40124 Bologna Tel. 051 6390906 - Fax 051 4210174 e-mail: [email protected] www.siesonline.it SEGRETERIA ORGANIZZATIVA Studio ER Congressi Via De' Poeti, 1/7 - 40124 Bologna Tel. 051 4210559 - Fax 051 4210174 e-mail: [email protected] www.ercongressi.it ABSTRACT BOOK supplement 2 - October 2020 Table of Contents XVI Congress of the Italian Society of Experimental Hematology Napoli, Italy, October 15-17, 2020 Main Program . 1 Best Abstracts . 20 Oral Communications Session 1. C001-C008 Acute Leukemia 1 . 23 Session 2. C009-C016 Chronic Lymphocytic Leukemia 1 . 28 Session 3. C017-C024 Multiple Myeloma 1 . 32 Session 4. C025-C032 Benign Hematology . 36 Session 5. C033-C040 Multiple Myeloma 2 . 42 Session 6. C041-C048 Acute Leukemia 2 . 45 Session 7. C049-C056 Molecular Hematology . 50 Session 8. C057-C064 Lymphomas. 54 Session 9. C065-C072 Chronic Lymphocytic Leukemia 2 . 57 Session 10. C073-C080 Myelodisplastic Syndromes and Acute Leukemia . 62 Session 11. C081-C088 Myeloproliferative Disorders and Chronic Myeloid Leukemia . 66 Session 12. C089-C096 Stem Cell Transplantation. 71 Posters Session 1. P001 Stem cells and growth factors . -
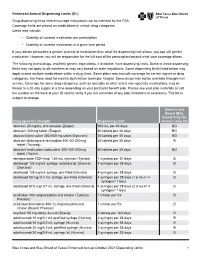
Enhanced Annual Drug List Dispensing Limits
Enhanced Annual Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral solution (Ziagen) 960 mL per 30 days BG abacavir 300 -

University of Groningen Multi-Residue Analysis of Growth Promotors In
University of Groningen Multi-residue analysis of growth promotors in food-producing animals Koole, Anneke IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 1998 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Koole, A. (1998). Multi-residue analysis of growth promotors in food-producing animals. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 25-09-2021 APPENDIX 1 OVERVIEW OF RELEVANT SUBSTANCES This appendix consists of two parts. First, substances that are relevant for the research presented in this thesis are given. For each substance CAS number (CAS), molecular weight (MW), bruto formula (formula) and if available UV maxima and alternative names are given. In addition, pKa values for the ß-agonists are listed, if they were available.