Assessment of a Spodumene Ore by Advanced Analytical and Mass Spectrometry Techniques to Determine Its Amenability to Processing for the Extraction of Lithium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
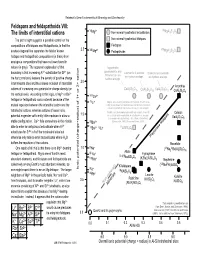
Feldspars and Feldspathoids VIII: the Limits of Interstitial Cations
Railsback's Some Fundamentals of Mineralogy and Geochemistry Feldspars and feldspathoids VIII: VI 2+ VI The limits of interstitial cations Mg Non-mineral hypothetical tectosilicates MgAl2Si2O8 The plot at right suggests a possible control on the Non-mineral hypothetical feldspars compositions of feldspars and feldspathoids, in that the Feldspars 2.5 VIII 2+ VIII dashed diagonal line separates the field of known Mg Feldspathoids MgAl2Si2O8 feldspar and feldspathoid compositions (in black) from analogous compositions that have not been found in nature (in gray). The apparent explanation of that Yugawaralite, goosecreekite, and boundary is that increasing Al3+ substtiution for Si4+ (on Laumontite & wairikite Scolecite and cowlesite tschernichite are are hydrous analogs. are hydrous analogs. the horizontal axis) lessens the density of positive charge hydrous analogs. in tetrahedral sites and thus allows inclusion of interstitial 2.0 Anorthite cations of increasing ionic potential or charge density (on CaAl2Si6O16 CaAl Si O CaAl Si O 2 4 12 2 3 10 CaAl2Si2O8 the vertical axis). According to this logic, a Mg2+ or Be2+ VIIICa2+ feldspar or feldspathoid could not exist because of the IVLi+ Virgilite, an Li-bearing anhydrous tectosilicate (French et al., mutual repulsion between the interstitial cation and the 1978) is not shown here both because its chemical formula is not well constrained and because it is a very rare mineral. tetrahedral cations, whereas cations of lesser ionic Petalite, an Li-bearing anhydrous silicate in which Al and Si Celsian potential engender sufficiently little repulsion to allow a 1.5 are in tetrahedral coordination, is not shown here because it is considered a phyllosilicate rather than a tectosilicate BaAl2Si2O8 stable configuration. -

Rubidium-Rich Feldspars and Associated Minerals from the Luolamäki Pegmatite, Somero, Finland
RUBIDIUM-RICH FELDSPARS AND ASSOCIATED MINERALS FROM THE LUOLAMÄKI PEGMATITE, SOMERO, FINLAND DAVID K. TEERTSTRA, PETR ÖERNY and FRANK C. HAWTHORNE TEERTSTRA, D.K., CERNY, P. and HAWTHORNE, F.C. 1998. Rubidium-rich feldspars and associated minerals from the Luolamäki pegmatite, Somero, Fin- land. Bulletin of the Geological Society of Finland 70, Parts 1-2, 43^f9. Rubidium feldspar occurs near the core zone of the highly fractionated pet- alite-subtype Luolamäki granitic pegmatite in intimate intergrowth with other feldspars which are part of a characteristic sequence of alteration of pollucite. Pods of pollucite are cut by 5-20 cm-wide veins of albite, petalite, non-perthit- ic microcline, lepidolite and quartz, by thinner veins of fine-grained micas and spodumene, and are replaced by metasomatic adularia. Grains of rubidium feld- spar occur as a potentially ordered phase in the vein microcline in association with earlier-exsolved albite, and also as late thin (< 5 |J.m) veinlets. Rubidium feldspar also occurs as a potentially disordered phase which crystallized along with metasomatic adularia. Both generations of (Rb,K)-feldspar have a similar compositional range, close to the join KAlSi308-RbAlSi308, typically with up to -21 wt.% Rb,0 (-70 mol.% Rbf) and with minor Cs, but neglible Na, Ca, Fe or P. Extreme compositions have 26.0 wt.% Rb,0 (89.0 mol.% Rbf) and 1.26 wt.% Cs20 (2.8 mol.% Csf). The diffuse compositional gradients from micro- cline to rubicline are consistent with a solid-state exsolution origin, followed by fluid-assisted textural coarsening which generates distinct phase boundaries. -

Italian Type Minerals / Marco E
THE AUTHORS This book describes one by one all the 264 mi- neral species first discovered in Italy, from 1546 Marco E. Ciriotti was born in Calosso (Asti) in 1945. up to the end of 2008. Moreover, 28 minerals He is an amateur mineralogist-crystallographer, a discovered elsewhere and named after Italian “grouper”, and a systematic collector. He gradua- individuals and institutions are included in a pa- ted in Natural Sciences but pursued his career in the rallel section. Both chapters are alphabetically industrial business until 2000 when, being General TALIAN YPE INERALS I T M arranged. The two catalogues are preceded by Manager, he retired. Then time had come to finally devote himself to his a short presentation which includes some bits of main interest and passion: mineral collecting and information about how the volume is organized related studies. He was the promoter and is now the and subdivided, besides providing some other President of the AMI (Italian Micromineralogical As- more general news. For each mineral all basic sociation), Associate Editor of Micro (the AMI maga- data (chemical formula, space group symmetry, zine), and fellow of many organizations and mine- type locality, general appearance of the species, ralogical associations. He is the author of papers on main geologic occurrences, curiosities, referen- topological, structural and general mineralogy, and of a mineral classification. He was awarded the “Mi- ces, etc.) are included in a full page, together cromounters’ Hall of Fame” 2008 prize. Etymology, with one or more high quality colour photogra- geoanthropology, music, and modern ballet are his phs from both private and museum collections, other keen interests. -

Refinement of the Crystal Structure of a Synthetic Non-Stoichiometric Rb
Mineralogical Magazine, August 2001, Vol. 65(4), pp. 523–531 Refinement of the crystalstructure of asyntheticnon-stoichiometricRb-feldspar A. KYONO* AND M. KIMATA Institute of Geoscience, University of Tsukuba, Tennodai 1-1-1, Tsukuba, Ibaraki, 305-8571, Japan ABSTR ACT Thecrystal structure of hydrothermally synthesized Rb-feldspar (monoclinic, space group C2/m, a = 8.839(2) AÊ , b =13.035(2)A Ê , c = 7.175(2) AÊ , b = 116.11(1)8, V = 742.3(3) AÊ 3, Z =4)hasbeen refined to a final R of0.0574 for 692 independent X -rayreflections. Microprobe analyses of the Rb-feldspar suggestdeviation from stoichiometry, with excess Si andAl, resulting in a unitformula of Rb0.811&0.127Al1.059Si3.003O8.Infrared(IR) spectra indicate the structural occupancy of large H 2O content,which implies that the &Si4O8 substitutionfavours the structural incorporation of the H 2O moleculeat the M-site.The mean T Odistancesare 1. 632A Ê for T1 and 1.645 AÊ for T2,revealing highlydisordered (Al,Si) distribution with Al/ Si =0.245/0.755( T1site)and 0. 255/0.745( T2 site). Thereare two geochemical implications from this refinement: (1) identification of both rubicline triclinicwith (Al,Si) ordered distribution and synthetic monoclinic RbAlSi 3O8 with(Al,Si) disordered distributionimplies that Rb cannot be one of factors disrupting the (Al,Si) ordered and disordered distributionsin feldspars; and (2) natural and synthetic feldspars capable of accommodating the large cationstend to incorporate &Si4O8,excessAl andH 2Ocomponentsin their crystal structures. KEY WORDS: Rb-feldspar, -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

Rubicline, a New Feldspar from San Piero in Campo, Elba, Italy
American Mineralogist, Volume 83, pages 1335±1339, 1998 Rubicline, a new feldspar from San Piero in Campo, Elba, Italy DAVID K. TEERTSTRA,1 PETR CÏ ERNYÂ ,1 FRANK C. HAWTHORNE,1,* JULIE PIER,2 LU-MIN WANG,2,² and RODNEY C. EWING2,² 1Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba, Canada R3T 2N2 2Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, New Mexico 87131-1116, U.S.A. ABSTRACT The rubidium analogue of microcline, rubicline, (Rb,K)AlSi3O8, ideally RbAlSi3O8, was found in a pollucite-bearing rare-element pegmatite at San Piero in Campo, Elba, Italy. Rubicline is the ®rst mineral with rubidium as an essential constituent. It occurs as abundant but small (#50 mm) rounded grains in 1±2 cm wide veins of rubidian microcline (6 albite, muscovite, quartz, and apatite) that crosscut pollucite. Rubicline is brittle, transparent, and colorless. Refractive indices are slightly higher than those of the host microcline. The birefringence is low (1st order gray interference colors), and crys- tals are apparently untwinned. In thin and polished sections, cleavage passes through both the host microcline and grains of rubicline; by analogy with microcline, the cleavage is {001} perfect and {010} good. Determination of additional physical properties is hindered by an average grain size of 20 mm, heterogeneous composition, and structural coherency of rubicline with the enveloping microcline. Rubicline is triclinic, probable space group P1, with a 5 8.81(3), b 5 13.01(3) c 5 7.18(4) AÊ , a590.3(1), b5 115.7(3), g588.2(1)8, V 5 741 AÊ 3,Z5 4, and axial ratios a:b:c of 0.677:1:0.577 (calculated from electron-diffraction data). -

Ab Initio Calculation of Equilibrium Isotopic Fractionations of Potassium and Rubidium in Minerals and Water † ‡ ‡ † † § ‡ ∥ ⊥ Hao Zeng, , Viktor F
Article Cite This: ACS Earth Space Chem. 2019, 3, 2601−2612 http://pubs.acs.org/journal/aesccq Ab Initio Calculation of Equilibrium Isotopic Fractionations of Potassium and Rubidium in Minerals and Water † ‡ ‡ † † § ‡ ∥ ⊥ Hao Zeng, , Viktor F. Rozsa, Nicole Xike Nie, Zhe Zhang, Tuan Anh Pham, Giulia Galli, , , † and Nicolas Dauphas*, † ‡ Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, and Pritzker School of Molecular Engineering, The University of Chicago, Chicago, Illinois 60637, United States § Quantum Simulations Group, Lawrence Livermore National Laboratory, Livermore, California 94551, United States ∥ Materials Science Division, Argonne National Laboratory, Argonne, Illinois 60439, United States ⊥ Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States *S Supporting Information ABSTRACT: We used first-principle approaches to calculate the equilibrium isotopic fractionation factors of potassium (K) and rubidium (Rb) in a variety of minerals of geological relevance (orthoclase, albite, muscovite, illite, sylvite, and phlogopite). We also used molecular dynamics simulations to calculate the equilibrium isotopic fractionation factors of K in water. Our results indicate that K and Rb form bonds of similar strengths and that the ratio between the equilibrium fractionations of K and Rb is approximately 3−4. Under low- temperature conditions relevant to weathering of continents or alteration of seafloor basalts (∼25 °C), the K isotopic fractionation between solvated K+ and illite (a proxy for K-bearing clays) is +0.24‰, exceeding the current analytical precision, so equilibrium isotopic fractionation can induce measurable isotopic fractionations for this system at low temperature. These findings, however, cannot easily explain why the δ41K value of seawater is shifted by +0.6‰ relative to igneous rocks. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal Permalink https://escholarship.org/uc/item/9m86t9pq Author Daniels, JILLIAN Publication Date 2016 Supplemental Material https://escholarship.org/uc/item/9m86t9pq#supplemental Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal A thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Geology by JILLIAN THERESA Daniels 2016 ABSTRACT OF THE THESIS Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal by JILLIAN THERESA Daniels Master of Science in Geology University of California, Los Angeles, 2016 Professor Kevin D McKeegan, Chair The luminescence characteristics of a variety of feldspars were investigated using the post-IR IRSL signal to facilitate the understanding of the origins and behavior of signals used in luminescence dating. These feldspars were characterized in terms of major composition, Al,Si ordering, and minor impurities, and compared to the luminescence decay parameters of brightness, bleachability, and kinetic order calculated from the pIRIR decay signal. The behavior of these parameters over varying preheat temperatures, pIRIR measurement temperatures, IR50 measurement times, and first IR measurement temperatures were analyzed. -
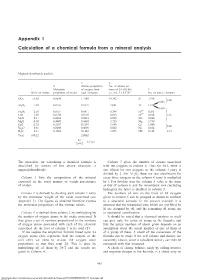
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Rubicline, a New Feldspar from San Piero in Campo, Elba, Italy
AmericanMineralogist, Volume 83,pages 1335-1339,1998 Rubicline, a new feldspar from San Piero in Campo, Elba, Italy Devro K. Tnpnrsrnrrr PerR dnnNfr'FruNr C. HlwrnoRNErr.* Jur-rc PrER,2 Lu-MrN WaNcr2't and RooNBy C. EwrNcr't IDepartment of Geological Sciences,University of Manitoba, Winnipeg, Manitoba, CanadaR3T 2N2 '?Deparlment of Earth and PlanetarySciences, University of New Mexico, Albuquerque,New Mexico 87131-1116,U S A. Ansrnlcr The rubidium analogueof microcline, rubicline, (Rb,K)AlSi3O,, ideally RbAlSi3Os, was found in a pollucite-bearing rare-element pegmatite at San Piero in Campo, Elba, Italy. Rubicline is the first mineral with rubidium as an essential constituent. It occurs as abundantbut small (<50 pm) rounded grains in r-2 cm wide veins of rubidian microcline (+ albite, muscovite, quartz, and apatite) that crosscut pollucite. Rubicline is brittle, transparent,and colorless. Refractive indices are slightly higher than those of the host microcline. The birefringenceis low (1st order gray interferencecolors), and crys- tals are apparently untwinned. In thin and polished sections, cleavage passes through both the host microcline and grains of rubicline; by analogy with microcline, the cleavage is {001} perfect and {010} good. Determination of additional physical properties is hindered by an average grain size of 20 p,m, heterogeneouscomposition, and structural coherency of rubicline with the enveloping microcline. Rubicline is triclinic, probable spacegroup Pl, with a : 8.81(3),b : 13.01(3)c : 7.18(4)A, : 90.3(1), : " B 115.7(3),"y : 88.2(1)', V = 741 A', Z : 4, and axial ratios a:b:c of 0.6jj:l:0.57'l (calculated from electron-diffraction data). -

Technical Report 2020 Update on the Pajarito Gold / Silver Property, Morelos Municipality, Chihuahua, Mexico
TECHNICAL REPORT 2020 UPDATE ON THE PAJARITO GOLD / SILVER PROPERTY, MORELOS MUNICIPALITY, CHIHUAHUA, MEXICO Latitude 26o25’41.4” N, Longitude -107o54’37.7” Prepared for: Grupo Minero Diflor S.A. de C.V. Montana 22 Col. Nápoles Delegación Benito Juárez México City, México C.P. 03810 William Feyerabend CPG - 11047 April 13, 2020 Pajarito Gold and Silver Property, Chihuahua, Mexico DATE AND SIGNATURE PAGE I, William Feyerabend, do certify that: 1) I am a consulting geologist located at 4218 Kachina Way, Prescott Valley, AZ 86314 2) The title of this report is “Technical Report 2019 Update on the Pajarito Gold Property, Morelos Municipality, Chihuahua, Mexico” dated April 13, 2020. 3) I graduated with a Bachelor of Science degree from the University of Southern California in 1972. I am a member in good standing of the American Institute of Professional Geologists. I have worked as a geologist for over 30 years since my graduation from university. That experience includes over twenty five years of exploration and development for gold/silver on four continents. I meet the definition of Qualified Person for the purposed of this instrument . 4) The current report is based on the last of multiple field visits from February 3 thru February 5, 2020. 5) I am responsible for the entire contents of this report. 6) I am independent of Grup Minero Diflor applying all of the tests in Section 1.5 of NI 43-101. 7) I have read NI 43-101 and Form 43-101F1, and this Technical Report has been prepared in compliance with that instrument and form. -

1 Encyclopedia of Mineral Names: First
1 ENCYCLOPEDIA OF MINERAL NAMES: FIRST UPDATE ROBERT F. MARTIN§ Department of Earth and Planetary Sciences, McGill University, 3450 University Street, Montreal, Quebec H3A 2A7, Canada WILLIAM H. BLACKBURN§ Royal Roads University, 2005 Sooke Road, Victoria, British Columbia V9B 5Y2, Canada § E-mail addresses: [email protected], [email protected] We are pleased to present the first update to the Encyclopedia of Mineral Names (The Canadian Mineralogist, Special Publication 1). The entries listed below largely consist of new species of minerals that have appeared in the literature since mid-1997, the date of publication of the Encyclopedia. A copy of this update will be supplied free of charge on demand to those who already own the Encyclopedia, and will be included with all copies of the first edition of the Encyclopedia sold henceforth. The information presented below is presented in five separate lists. First is the listing of new mineral species discovered since the Encyclopedia appeared two years ago. The second list shows the new species defined as a result of decisions summarized in the IMA report on micas (Can. Mineral. 36, 905, 1998). Thirdly, we present the listing of new species defined as a result of decisions summarized in the IMA report on zeolite-group minerals (Can. Mineral. 35, 1571, 1997). Fourthly, we present a short list of mineral species discovered long ago that were omitted from the first edition of the Encyclopedia. Lastly, we present minerals already in the Encyclopedia that have been discredited as valid species since 1997, or that were misspelled. We acknowledge the assistance of many readers who took the trouble to offer corrections, not only to the names of mineral species, but also to names of localities.