1 Encyclopedia of Mineral Names: First
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Studies of the Zeolites Composition of Zeolites of the Natrolite Group and Compositional Relations Among Thomsonites Gonnardites, and Natrolites
r-'1 ~ Q I ~ c lt') ~ r-'1 'JJ ~ Q.) < ~ ~ ......-~ ..,.;;j ~ <z 0 0 Q.) 1-4 rJ:J rJ:J N r-'1 ~ Q.) 0 ~ ..c ~ ~ ~ I r-'1 ~ > ~ 0 I ~ rJ:J 'JJ ..,.;;j Q.) < .....-~ 0 . 1-4 ~ C"-' 0 ~ ..,.;;j ~ 0 r-'1 00 C"-' Foster-STUDIES•. OF THE ZEOLITES-Geological Survey Professional Paper 504-D, E Studies of the Zeolites Composition of Zeolites of the Natrolite Group and Compositional Relations among Thomsonites Gonnardites, and Natrolites By MARGARET D. FOSTER SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 504-D, E UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1965 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretory GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publication as follows: Foster, Margaret Dorothy, 1895- Studies of the zeolites. D. Composition of zeolites of the natrolite group. E. Compositional relations among thom sonites, gonnardites, and natrolites. Washington, U.S. Govt. Print. Off., 1965. v, 7; iii, 10 p. diagrs., tables. 30 em. (U.S. Geological Survey. Professional paper 504-D, E) Shorter contributions to general geology. Each part also has separate title page. Includes bibliographies. (Continued on next card) Foster, Margaret Dorothy, 1895- Studies of the zeolites. 1965. (Card 2) 1. Zeolites. I. Title. II. Title: Composition of zeolites of the natro lite group. III. Title: Compositional relations among thomsonites, gonnardites, and natrolites. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 25 cents (paper cover) Studies of the Zeolites Composition of Zeolites of the N atrolite Group By MARGARET D. -

Third-Generation Synchrotron X-Ray Diffraction of 6- M Crystal of Raite, Na
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 12263–12267, November 1997 Geology Third-generation synchrotron x-ray diffraction of 6-mm crystal of raite, 'Na3Mn3Ti0.25Si8O20(OH)2z10H2O, opens up new chemistry and physics of low-temperature minerals (crystal structureymicrocrystalyphyllosilicate) JOSEPH J. PLUTH*, JOSEPH V. SMITH*†,DMITRY Y. PUSHCHAROVSKY‡,EUGENII I. SEMENOV§,ANDREAS BRAM¶, CHRISTIAN RIEKEL¶,HANS-PETER WEBER¶, AND ROBERT W. BROACHi *Department of Geophysical Sciences, Center for Advanced Radiation Sources, GeologicalySoilyEnvironmental, and Materials Research Science and Engineering Center, 5734 South Ellis Avenue, University of Chicago, Chicago, IL 60637; ‡Department of Geology, Moscow State University, Moscow, 119899, Russia; §Fersman Mineralogical Museum, Russian Academy of Sciences, Moscow, 117071, Russia; ¶European Synchrotron Radiation Facility, BP 220, 38043, Grenoble, France; and UOP Research Center, Des Plaines, IL 60017 Contributed by Joseph V. Smith, September 3, 1997 ABSTRACT The crystal structure of raite was solved and the energy and metal industries, hydrology, and geobiology. refined from data collected at Beamline Insertion Device 13 at Raite lies in the chemical cooling sequence of exotic hyperal- the European Synchrotron Radiation Facility, using a 3 3 3 3 kaline rocks of the Kola Peninsula, Russia, and the 65 mm single crystal. The refined lattice constants of the Monteregian Hills, Canada (2). This hydrated sodium- monoclinic unit cell are a 5 15.1(1) Å; b 5 17.6(1) Å; c 5 manganese silicate extends the already wide range of manga- 5.290(4) Å; b 5 100.5(2)°; space group C2ym. The structure, nese crystal chemistry (3), which includes various complex including all reflections, refined to a final R 5 0.07. -

Gonnardite Na2caal4si6o20 ² 7H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Tetragonal
Gonnardite Na2CaAl4Si6O20 ² 7H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Tetragonal. Point Group: 42m: Fibrous crystals, at the centers of radiating spherulites, to 3 cm; massive. Physical Properties: Hardness = 5 D(meas.) = 2.25{2.36 D(calc.) = 2.33 Optical Properties: Translucent. Color: White, yellowish to salmon-red. Luster: Silky. Optical Class: Biaxial (+) or ({); commonly zoned. Orientation: X = c. ® = 1.497{1.508 ¯ = 1.498{1.510 ° = 1.499{1.513 2V(meas.) = 50± Cell Data: Space Group: I42d: a = 13.21(1) c = 6.622(4) Z = 2 X-ray Powder Pattern: Chaux de Bergonne, France; may be confused with natrolite and tetranatrolite. 2.92 (100), 5.93 (80), 6.70 (60), 4.44 (60), 4.74 (50), 3.23 (50), 3.12 (40) Chemistry: (1) (2) (3) SiO2 43.45 43.20 44.58 Al2O3 27.91 27.90 25.22 CaO 6.95 3.61 6.94 Na2O 8.69 13.16 7.66 H2O [13.00] 11.74 15.60 Total [100.00] 99.61 100.00 (1) Chaux de Bergonne, France; by electron microprobe, H2O by di®erence; corresponding to Na2:22Ca0:98Al4:32Si5:71O20 ² 5:70H2O: (2) Aci Trezza, Sicily, Italy; corresponds to Na3:5Ca0:5Al4:5 Si5:9O20:8 ² 5:35H2O: (3) Na2CaAl4Si6O20 ² 7H2O: Mineral Group: Zeolite group. Occurrence: In cavities in basalt, leucite tephrite, and altered skarn. Association: Zeolites, calcite. Distribution: Well characterized material from: in France, at Chaux de Bergonne, Gignat, Puy de D^ome. In Italy, from Capo di Bove, near Rome, Lazio; and at Aci Castello, Aci Trezza, Osilo, and other places on Sardinia. -

Newsletter June 2006
w NEWSLETTER INTERNATIONAL TUNGSTEN INDUSTRY ASSOCIATION Rue Père Eudore Devroye 245, 1150 Brussels, Belgium Tel: +32 2 770 8878 Fax: + 32 2 770 8898 E-mail: [email protected] Web: www.itia.info The programme will begin on Tuesday Toxicologic Assessment for the Production and 19th evening with a Reception in the hotel jointly Mechanical Processing of Materials containing hosted by Tiberon Minerals and ITIA. Tungsten Heavy Metal. Not least will be an introduction to the formation of a Consortium to deal Working sessions will be held on Wednesday Annual with the implications of REACH on behalf of the and Thursday mornings and papers will tungsten industry, both legal and financial. include: General Readers will recall that REACH will place an obligation ▼ HSE work programme on individual companies to submit a technical dossier and register any chemical substance Meeting ▼ Ganzhou’s Tungsten Industry and Its Progress manufactured or imported into the EU in quantities ▼ Geostatistics in the mid and long-term planning for of more than 1 tonne. the Panasqueira deposit (Nuno Alves, Beralt Tin Tuesday 26 to and Wolfram) In order to assist companies with the registration process, the Commission recommends the creation ▼ Thursday 28 Paper by Zhuzhou Cemented Carbide of industry Consortia. These Consortia will enable the ▼ Update on Tiberon’s Development of Tungsten joint development, submission and sharing of September 2006 Mining in Vietnam information with the aim of reducing the compliance burden on individual companies and preventing ▼ Update on China’s Tungsten Industry Hyatt Regency Hotel, unnecessary additional animal testing. ▼ Update on US Tungsten Market (Dean Schiller, Boston, USA OsramSylvania Products) Both members and non-members will be equally ▼ Review of Trends in 2006 (Nigel Tunna, Metal-Pages) welcome to join the Consortium, saving themselves considerable amounts of money and time by so doing. -
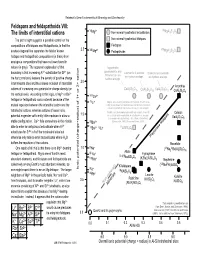
Feldspars and Feldspathoids VIII: the Limits of Interstitial Cations
Railsback's Some Fundamentals of Mineralogy and Geochemistry Feldspars and feldspathoids VIII: VI 2+ VI The limits of interstitial cations Mg Non-mineral hypothetical tectosilicates MgAl2Si2O8 The plot at right suggests a possible control on the Non-mineral hypothetical feldspars compositions of feldspars and feldspathoids, in that the Feldspars 2.5 VIII 2+ VIII dashed diagonal line separates the field of known Mg Feldspathoids MgAl2Si2O8 feldspar and feldspathoid compositions (in black) from analogous compositions that have not been found in nature (in gray). The apparent explanation of that Yugawaralite, goosecreekite, and boundary is that increasing Al3+ substtiution for Si4+ (on Laumontite & wairikite Scolecite and cowlesite tschernichite are are hydrous analogs. are hydrous analogs. the horizontal axis) lessens the density of positive charge hydrous analogs. in tetrahedral sites and thus allows inclusion of interstitial 2.0 Anorthite cations of increasing ionic potential or charge density (on CaAl2Si6O16 CaAl Si O CaAl Si O 2 4 12 2 3 10 CaAl2Si2O8 the vertical axis). According to this logic, a Mg2+ or Be2+ VIIICa2+ feldspar or feldspathoid could not exist because of the IVLi+ Virgilite, an Li-bearing anhydrous tectosilicate (French et al., mutual repulsion between the interstitial cation and the 1978) is not shown here both because its chemical formula is not well constrained and because it is a very rare mineral. tetrahedral cations, whereas cations of lesser ionic Petalite, an Li-bearing anhydrous silicate in which Al and Si Celsian potential engender sufficiently little repulsion to allow a 1.5 are in tetrahedral coordination, is not shown here because it is considered a phyllosilicate rather than a tectosilicate BaAl2Si2O8 stable configuration. -

Subject Index, Volume 83, 1998
American Mineralogist, Volume 83, pages 1377–1385, 1998 SUBJECT INDEX, VOLUME 83, 1998 Actinolite 458 jadeitic pyroxene 273 Arsenic model compounds 553 AFM lithium disilicate 1008 Arsenopyrite 316 illite-smectite 762 magnesiowüstite 794 ASH system 881 nucleation and growth 147 MgMgAl-pumpellyite 220 Atmospheric CO2 1503 2+ 3+ Agrell, Stuart O., memorial of 666 Mn (Fe, Mn) 2 O4 786 Augite 419, 434 Albite glass 1141 monazite 248 Awards Al-Fe perovskite (MgSiO3) 947 monazite-(Ce) 259 Mineralogical Society of America, ac - Allanite 248 muscovite 535 ceptance of 917 Allende meteorite 970 muscovite-phengite 775 Mineralogical Society of America, pre- Almandite 1293 offretite 577 sentation of 916 27Al MAS NMR olivine 546 Roebling Medal, acceptance of 914 Al2O3+B2O3+SiO2 ± H2O 638 omphacite 419 Roebling Medal, presentation of 912 AlSiO3OH 881 paranite-(Y) 1100 B8/anti-B8 451 Ammonium illilte 58 phase egg 881 B8-FeO 451 Analcime 339, 746 phosphovanadylilte 889 Bacteria 1583 Analysis, chemical (mineral) pyrope 323, 1293 Bacterial surfaces 1399 almandite 1293 pyroxene 491 Bacterial synthesis of greigite 1469 andradite 835 rossmanite 896 Band Gap 865 anorthite 1209 rubicline 1335 Bamfordite 172 apatite 240, 1122 scandiobabingtonite 1330 Basaltic andesite 36 arsenopyrite 316 scheelite 1100 BaTi5Fe6MgO19 1323 augite 419 schorl 848 Benyacarite 400 bamfordite 172 sodic-ferri-clinoferroholmquistite 668 Berezanskite 907 biogenic magnetite 1409 sorosite 901 Bioaccumulation 1503 boralsilite 638 spessartine 1293 Biogenic magnetite 1409 bornite 1231 titanite 1168 Biogeochemistry 1418, 1494, 1593, 1426 brabantite 259 tourmaline 535, 638, 848 Biomineralization 1454, 1510 brucite 68 tremolite–actinolite–ferro-actinolite 458 Birnessite 305 buergerite 848 tschörtnerite 607 Boehmite 1209 Ca-amphibole 952 wüstite 451 Book reviews caleite 1510, 1503 xenotime 1302 Carey, J.William: Thermodynamics carbon 918 yvonite 383 of Natural Systems. -

Rubidium-Rich Feldspars and Associated Minerals from the Luolamäki Pegmatite, Somero, Finland
RUBIDIUM-RICH FELDSPARS AND ASSOCIATED MINERALS FROM THE LUOLAMÄKI PEGMATITE, SOMERO, FINLAND DAVID K. TEERTSTRA, PETR ÖERNY and FRANK C. HAWTHORNE TEERTSTRA, D.K., CERNY, P. and HAWTHORNE, F.C. 1998. Rubidium-rich feldspars and associated minerals from the Luolamäki pegmatite, Somero, Fin- land. Bulletin of the Geological Society of Finland 70, Parts 1-2, 43^f9. Rubidium feldspar occurs near the core zone of the highly fractionated pet- alite-subtype Luolamäki granitic pegmatite in intimate intergrowth with other feldspars which are part of a characteristic sequence of alteration of pollucite. Pods of pollucite are cut by 5-20 cm-wide veins of albite, petalite, non-perthit- ic microcline, lepidolite and quartz, by thinner veins of fine-grained micas and spodumene, and are replaced by metasomatic adularia. Grains of rubidium feld- spar occur as a potentially ordered phase in the vein microcline in association with earlier-exsolved albite, and also as late thin (< 5 |J.m) veinlets. Rubidium feldspar also occurs as a potentially disordered phase which crystallized along with metasomatic adularia. Both generations of (Rb,K)-feldspar have a similar compositional range, close to the join KAlSi308-RbAlSi308, typically with up to -21 wt.% Rb,0 (-70 mol.% Rbf) and with minor Cs, but neglible Na, Ca, Fe or P. Extreme compositions have 26.0 wt.% Rb,0 (89.0 mol.% Rbf) and 1.26 wt.% Cs20 (2.8 mol.% Csf). The diffuse compositional gradients from micro- cline to rubicline are consistent with a solid-state exsolution origin, followed by fluid-assisted textural coarsening which generates distinct phase boundaries. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Article Benefited from Construc- Ering the Co Dominance Among the Non-Cu Metal Atoms, Tive Reviews by Jochen Schlüter and Taras Panikorovskii
Eur. J. Mineral., 32, 637–644, 2020 https://doi.org/10.5194/ejm-32-637-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Gobelinite, the Co analogue of ktenasite from Cap Garonne, France, and Eisenzecher Zug, Germany Stuart J. Mills1, Uwe Kolitsch2,3, Georges Favreau4, William D. Birch1, Valérie Galea-Clolus5, and Johannes Markus Henrich6 1Geosciences, Museums Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia 2Mineralogisch-Petrographische Abt., Naturhistorisches Museum, Burgring 7, 1010 Vienna, Austria 3Institut für Mineralogie und Kristallographie, Universität Wien, Althanstraße 14, 1090 Vienna, Austria 4independent researcher: 421 Avenue Jean Monnet, 13090 Aix-en-Provence, France 5independent researcher: 10 rue Combe Noire, 83210 Solliès-Toucas, France 6independent researcher: Im Großen Garten 3, 57548 Kirchen (Sieg), Germany Correspondence: Stuart J. Mills ([email protected]) Received: 13 April 2020 – Revised: 30 October 2020 – Accepted: 9 November 2020 – Published: 25 November 2020 Abstract. The new mineral gobelinite, ideally CoCu4.SO4/2.OH/6 6H2O, is a new member of the ktenasite group and the Co analogue of ktenasite, ZnCu4.SO4/2.OH/6 6H2O.q It occurs at Cap Garonne (CG), Var, France (type locality), and Eisenzecher Zug (EZ), Siegerland, Northq Rhine-Westphalia, Germany (cotype lo- cality). The mineral forms pale green, bluish green or greyish green, blocky to thin, lath-like crystals. They are transparent and non-fluorescent, with a vitreous, sometimes also pearly, lustre and a white streak having a pale-green cast. Mohs hardness is about 2.5. The crystals are brittle with an irregular fracture; no cleav- age was observed. -

New Mineral Names*
American Mineralogist, Volume 84, pages 193±198, 1999 NEW MINERAL NAMES* JOHN L. JAMBOR1 AND ANDREW C. ROBERTS2 1Department of Earth Sciences, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada 2Geological Survey of Canada, 601 Booth Street, Ottawa K1A 0E8, Canada Arnhemite, pyrophosphite Arnhem Cave, 150 km east of Windhoek, Namibia. As- J.E.J. Martini (1994) Two new minerals originated from sociated minerals are minor archerite, small grains of bat guano combustion in Arnhem Cave, Namibia. Bull. quartz and cristobalite, and fragments of carbon. The slag South African Speleological Assoc., 33, 66±69. is interpreted to have resulted from the melting of ashes during the combustion of bat guano. Arnhemite formed Arnhemite subsequently by secondary hydration of an unknown pre- Occurs as ¯akes, up to 50 mm, that form aggregates cursor (see abstract for pyrocoproite). Arnhemite and pyr- making up the principal component of a thin layer of ophosphite are in the Transvaal Museum, Pretoria, South white, friable slag in a pro®le of cave-¯oor soil. White Africa. color, pearly luster, soft, perfect {001} cleavage, D 5 meas Discussion. Although data for arnhemite and pyro- 2.35, D 5 2.33 g/cm3 for Z 5 4. Insoluble in water, calc phosphite were submitted to the CNMMN prior to pub- readily soluble in HCl. Optically uniaxial negative, v5 lication of the paper, neither proposal was approved. 1.516, e51.503. The average and range of 12 electron J.L.J. microprobe analyses (H2O by gas chromatography) gave Na2O 4.48 (3.84±5.57), K2O 20.92 (17.50±22.03), MgO 11.31 (10.27±12.25), CaO 1.30 (0.88±2.46), MnO 0.28 (0.20±0.33), FeO 0.38 (0.07±0.67), ZnO 0.18 (0.08± Djer®sherite-thalfenisite analogs 0.41), P2O5 45.61 (43.95±46.94), H2O 14.70, sum 99.16 wt%, corresponding to (K Na ) (Mg Ca Fe A.Y. -

Calcium-Aluminum-Silicate-Hydrate “
Cent. Eur. J. Geosci. • 2(2) • 2010 • 175-187 DOI: 10.2478/v10085-010-0007-6 Central European Journal of Geosciences Calcium-aluminum-silicate-hydrate “cement” phases and rare Ca-zeolite association at Colle Fabbri, Central Italy Research Article F. Stoppa1∗, F. Scordari2,E.Mesto2, V.V. Sharygin3, G. Bortolozzi4 1 Dipartimento di Scienze della Terra, Università G. d’Annunzio, Chieti, Italy 2 Dipartimento Geomineralogico, Università di Bari, Bari, Italy 3 Sobolev V.S. Institute of Geology and Mineralogy, Siberian Branch of the Russian Academy of Sciences, Novosibirsk 630090, Russia 4 Via Dogali, 20, 31100-Treviso Received 26 January 2010; accepted 8 April 2010 Abstract: Very high temperature, Ca-rich alkaline magma intruded an argillite formation at Colle Fabbri, Central Italy, producing cordierite-tridymite metamorphism in the country rocks. An intense Ba-rich sulphate-carbonate- alkaline hydrothermal plume produced a zone of mineralization several meters thick around the igneous body. Reaction of hydrothermal fluids with country rocks formed calcium-silicate-hydrate (CSH), i.e., tobermorite- afwillite-jennite; calcium-aluminum-silicate-hydrate (CASH) – “cement” phases – i.e., thaumasite, strätlingite and an ettringite-like phase and several different species of zeolites: chabazite-Ca, willhendersonite, gismon- dine, three phases bearing Ca with the same or perhaps lower symmetry of phillipsite-Ca, levyne-Ca and the Ca-rich analogue of merlinoite. In addition, apophyllite-(KF) and/or apophyllite-(KOH), Ca-Ba-carbonates, portlandite and sulphates were present. A new polymorph from the pyrrhotite group, containing three layers of sphalerite-type structure in the unit cell, is reported for the first time. Such a complex association is unique.