UCLA Electronic Theses and Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
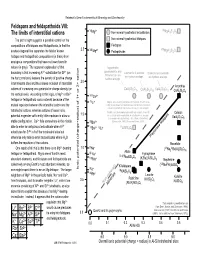
Feldspars and Feldspathoids VIII: the Limits of Interstitial Cations
Railsback's Some Fundamentals of Mineralogy and Geochemistry Feldspars and feldspathoids VIII: VI 2+ VI The limits of interstitial cations Mg Non-mineral hypothetical tectosilicates MgAl2Si2O8 The plot at right suggests a possible control on the Non-mineral hypothetical feldspars compositions of feldspars and feldspathoids, in that the Feldspars 2.5 VIII 2+ VIII dashed diagonal line separates the field of known Mg Feldspathoids MgAl2Si2O8 feldspar and feldspathoid compositions (in black) from analogous compositions that have not been found in nature (in gray). The apparent explanation of that Yugawaralite, goosecreekite, and boundary is that increasing Al3+ substtiution for Si4+ (on Laumontite & wairikite Scolecite and cowlesite tschernichite are are hydrous analogs. are hydrous analogs. the horizontal axis) lessens the density of positive charge hydrous analogs. in tetrahedral sites and thus allows inclusion of interstitial 2.0 Anorthite cations of increasing ionic potential or charge density (on CaAl2Si6O16 CaAl Si O CaAl Si O 2 4 12 2 3 10 CaAl2Si2O8 the vertical axis). According to this logic, a Mg2+ or Be2+ VIIICa2+ feldspar or feldspathoid could not exist because of the IVLi+ Virgilite, an Li-bearing anhydrous tectosilicate (French et al., mutual repulsion between the interstitial cation and the 1978) is not shown here both because its chemical formula is not well constrained and because it is a very rare mineral. tetrahedral cations, whereas cations of lesser ionic Petalite, an Li-bearing anhydrous silicate in which Al and Si Celsian potential engender sufficiently little repulsion to allow a 1.5 are in tetrahedral coordination, is not shown here because it is considered a phyllosilicate rather than a tectosilicate BaAl2Si2O8 stable configuration. -

Rubidium-Rich Feldspars and Associated Minerals from the Luolamäki Pegmatite, Somero, Finland
RUBIDIUM-RICH FELDSPARS AND ASSOCIATED MINERALS FROM THE LUOLAMÄKI PEGMATITE, SOMERO, FINLAND DAVID K. TEERTSTRA, PETR ÖERNY and FRANK C. HAWTHORNE TEERTSTRA, D.K., CERNY, P. and HAWTHORNE, F.C. 1998. Rubidium-rich feldspars and associated minerals from the Luolamäki pegmatite, Somero, Fin- land. Bulletin of the Geological Society of Finland 70, Parts 1-2, 43^f9. Rubidium feldspar occurs near the core zone of the highly fractionated pet- alite-subtype Luolamäki granitic pegmatite in intimate intergrowth with other feldspars which are part of a characteristic sequence of alteration of pollucite. Pods of pollucite are cut by 5-20 cm-wide veins of albite, petalite, non-perthit- ic microcline, lepidolite and quartz, by thinner veins of fine-grained micas and spodumene, and are replaced by metasomatic adularia. Grains of rubidium feld- spar occur as a potentially ordered phase in the vein microcline in association with earlier-exsolved albite, and also as late thin (< 5 |J.m) veinlets. Rubidium feldspar also occurs as a potentially disordered phase which crystallized along with metasomatic adularia. Both generations of (Rb,K)-feldspar have a similar compositional range, close to the join KAlSi308-RbAlSi308, typically with up to -21 wt.% Rb,0 (-70 mol.% Rbf) and with minor Cs, but neglible Na, Ca, Fe or P. Extreme compositions have 26.0 wt.% Rb,0 (89.0 mol.% Rbf) and 1.26 wt.% Cs20 (2.8 mol.% Csf). The diffuse compositional gradients from micro- cline to rubicline are consistent with a solid-state exsolution origin, followed by fluid-assisted textural coarsening which generates distinct phase boundaries. -

Italian Type Minerals / Marco E
THE AUTHORS This book describes one by one all the 264 mi- neral species first discovered in Italy, from 1546 Marco E. Ciriotti was born in Calosso (Asti) in 1945. up to the end of 2008. Moreover, 28 minerals He is an amateur mineralogist-crystallographer, a discovered elsewhere and named after Italian “grouper”, and a systematic collector. He gradua- individuals and institutions are included in a pa- ted in Natural Sciences but pursued his career in the rallel section. Both chapters are alphabetically industrial business until 2000 when, being General TALIAN YPE INERALS I T M arranged. The two catalogues are preceded by Manager, he retired. Then time had come to finally devote himself to his a short presentation which includes some bits of main interest and passion: mineral collecting and information about how the volume is organized related studies. He was the promoter and is now the and subdivided, besides providing some other President of the AMI (Italian Micromineralogical As- more general news. For each mineral all basic sociation), Associate Editor of Micro (the AMI maga- data (chemical formula, space group symmetry, zine), and fellow of many organizations and mine- type locality, general appearance of the species, ralogical associations. He is the author of papers on main geologic occurrences, curiosities, referen- topological, structural and general mineralogy, and of a mineral classification. He was awarded the “Mi- ces, etc.) are included in a full page, together cromounters’ Hall of Fame” 2008 prize. Etymology, with one or more high quality colour photogra- geoanthropology, music, and modern ballet are his phs from both private and museum collections, other keen interests. -
Gemstones and Geosciences in Space and Time Digital Maps to the “Chessboard Classification Scheme of Mineral Deposits”
Earth-Science Reviews 127 (2013) 262–299 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Gemstones and geosciences in space and time Digital maps to the “Chessboard classification scheme of mineral deposits” Harald G. Dill a,b,⁎,BertholdWeberc,1 a Federal Institute for Geosciences and Natural Resources, P.O. Box 510163, D-30631 Hannover, Germany b Institute of Geosciences — Gem-Materials Research and Economic Geology, Johannes-Gutenberg-University, Becherweg 21, D-55099 Mainz, Germany c Bürgermeister-Knorr Str. 8, D-92637 Weiden i.d.OPf., Germany article info abstract Article history: The gemstones, covering the spectrum from jeweler's to showcase quality, have been presented in a tripartite Received 27 April 2012 subdivision, by country, geology and geomorphology realized in 99 digital maps with more than 2600 mineral- Accepted 16 July 2013 ized sites. The various maps were designed based on the “Chessboard classification scheme of mineral deposits” Available online 25 July 2013 proposed by Dill (2010a, 2010b) to reveal the interrelations between gemstone deposits and mineral deposits of other commodities and direct our thoughts to potential new target areas for exploration. A number of 33 categories Keywords: were used for these digital maps: chromium, nickel, titanium, iron, manganese, copper, tin–tungsten, beryllium, Gemstones fl Country lithium, zinc, calcium, boron, uorine, strontium, phosphorus, zirconium, silica, feldspar, feldspathoids, zeolite, Geology amphibole (tiger's eye), olivine, pyroxenoid, garnet, epidote, sillimanite–andalusite, corundum–spinel−diaspore, Geomorphology diamond, vermiculite–pagodite, prehnite, sepiolite, jet, and amber. Besides the political base map (gems Digital maps by country) the mineral deposit is drawn on a geological map, illustrating the main lithologies, stratigraphic Chessboard classification scheme units and tectonic structure to unravel the evolution of primary gemstone deposits in time and space. -

STRONG and WEAK INTERLAYER INTERACTIONS of TWO-DIMENSIONAL MATERIALS and THEIR ASSEMBLIES Tyler William Farnsworth a Dissertati
STRONG AND WEAK INTERLAYER INTERACTIONS OF TWO-DIMENSIONAL MATERIALS AND THEIR ASSEMBLIES Tyler William Farnsworth A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2018 Approved by: Scott C. Warren James F. Cahoon Wei You Joanna M. Atkin Matthew K. Brennaman © 2018 Tyler William Farnsworth ALL RIGHTS RESERVED ii ABSTRACT Tyler William Farnsworth: Strong and weak interlayer interactions of two-dimensional materials and their assemblies (Under the direction of Scott C. Warren) The ability to control the properties of a macroscopic material through systematic modification of its component parts is a central theme in materials science. This concept is exemplified by the assembly of quantum dots into 3D solids, but the application of similar design principles to other quantum-confined systems, namely 2D materials, remains largely unexplored. Here I demonstrate that solution-processed 2D semiconductors retain their quantum-confined properties even when assembled into electrically conductive, thick films. Structural investigations show how this behavior is caused by turbostratic disorder and interlayer adsorbates, which weaken interlayer interactions and allow access to a quantum- confined but electronically coupled state. I generalize these findings to use a variety of 2D building blocks to create electrically conductive 3D solids with virtually any band gap. I next introduce a strategy for discovering new 2D materials. Previous efforts to identify novel 2D materials were limited to van der Waals layered materials, but I demonstrate that layered crystals with strong interlayer interactions can be exfoliated into few-layer or monolayer materials. -

Refinement of the Crystal Structure of a Synthetic Non-Stoichiometric Rb
Mineralogical Magazine, August 2001, Vol. 65(4), pp. 523–531 Refinement of the crystalstructure of asyntheticnon-stoichiometricRb-feldspar A. KYONO* AND M. KIMATA Institute of Geoscience, University of Tsukuba, Tennodai 1-1-1, Tsukuba, Ibaraki, 305-8571, Japan ABSTR ACT Thecrystal structure of hydrothermally synthesized Rb-feldspar (monoclinic, space group C2/m, a = 8.839(2) AÊ , b =13.035(2)A Ê , c = 7.175(2) AÊ , b = 116.11(1)8, V = 742.3(3) AÊ 3, Z =4)hasbeen refined to a final R of0.0574 for 692 independent X -rayreflections. Microprobe analyses of the Rb-feldspar suggestdeviation from stoichiometry, with excess Si andAl, resulting in a unitformula of Rb0.811&0.127Al1.059Si3.003O8.Infrared(IR) spectra indicate the structural occupancy of large H 2O content,which implies that the &Si4O8 substitutionfavours the structural incorporation of the H 2O moleculeat the M-site.The mean T Odistancesare 1. 632A Ê for T1 and 1.645 AÊ for T2,revealing highlydisordered (Al,Si) distribution with Al/ Si =0.245/0.755( T1site)and 0. 255/0.745( T2 site). Thereare two geochemical implications from this refinement: (1) identification of both rubicline triclinicwith (Al,Si) ordered distribution and synthetic monoclinic RbAlSi 3O8 with(Al,Si) disordered distributionimplies that Rb cannot be one of factors disrupting the (Al,Si) ordered and disordered distributionsin feldspars; and (2) natural and synthetic feldspars capable of accommodating the large cationstend to incorporate &Si4O8,excessAl andH 2Ocomponentsin their crystal structures. KEY WORDS: Rb-feldspar, -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

Rubicline, a New Feldspar from San Piero in Campo, Elba, Italy
American Mineralogist, Volume 83, pages 1335±1339, 1998 Rubicline, a new feldspar from San Piero in Campo, Elba, Italy DAVID K. TEERTSTRA,1 PETR CÏ ERNYÂ ,1 FRANK C. HAWTHORNE,1,* JULIE PIER,2 LU-MIN WANG,2,² and RODNEY C. EWING2,² 1Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba, Canada R3T 2N2 2Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, New Mexico 87131-1116, U.S.A. ABSTRACT The rubidium analogue of microcline, rubicline, (Rb,K)AlSi3O8, ideally RbAlSi3O8, was found in a pollucite-bearing rare-element pegmatite at San Piero in Campo, Elba, Italy. Rubicline is the ®rst mineral with rubidium as an essential constituent. It occurs as abundant but small (#50 mm) rounded grains in 1±2 cm wide veins of rubidian microcline (6 albite, muscovite, quartz, and apatite) that crosscut pollucite. Rubicline is brittle, transparent, and colorless. Refractive indices are slightly higher than those of the host microcline. The birefringence is low (1st order gray interference colors), and crys- tals are apparently untwinned. In thin and polished sections, cleavage passes through both the host microcline and grains of rubicline; by analogy with microcline, the cleavage is {001} perfect and {010} good. Determination of additional physical properties is hindered by an average grain size of 20 mm, heterogeneous composition, and structural coherency of rubicline with the enveloping microcline. Rubicline is triclinic, probable space group P1, with a 5 8.81(3), b 5 13.01(3) c 5 7.18(4) AÊ , a590.3(1), b5 115.7(3), g588.2(1)8, V 5 741 AÊ 3,Z5 4, and axial ratios a:b:c of 0.677:1:0.577 (calculated from electron-diffraction data). -

Ab Initio Calculation of Equilibrium Isotopic Fractionations of Potassium and Rubidium in Minerals and Water † ‡ ‡ † † § ‡ ∥ ⊥ Hao Zeng, , Viktor F
Article Cite This: ACS Earth Space Chem. 2019, 3, 2601−2612 http://pubs.acs.org/journal/aesccq Ab Initio Calculation of Equilibrium Isotopic Fractionations of Potassium and Rubidium in Minerals and Water † ‡ ‡ † † § ‡ ∥ ⊥ Hao Zeng, , Viktor F. Rozsa, Nicole Xike Nie, Zhe Zhang, Tuan Anh Pham, Giulia Galli, , , † and Nicolas Dauphas*, † ‡ Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, and Pritzker School of Molecular Engineering, The University of Chicago, Chicago, Illinois 60637, United States § Quantum Simulations Group, Lawrence Livermore National Laboratory, Livermore, California 94551, United States ∥ Materials Science Division, Argonne National Laboratory, Argonne, Illinois 60439, United States ⊥ Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States *S Supporting Information ABSTRACT: We used first-principle approaches to calculate the equilibrium isotopic fractionation factors of potassium (K) and rubidium (Rb) in a variety of minerals of geological relevance (orthoclase, albite, muscovite, illite, sylvite, and phlogopite). We also used molecular dynamics simulations to calculate the equilibrium isotopic fractionation factors of K in water. Our results indicate that K and Rb form bonds of similar strengths and that the ratio between the equilibrium fractionations of K and Rb is approximately 3−4. Under low- temperature conditions relevant to weathering of continents or alteration of seafloor basalts (∼25 °C), the K isotopic fractionation between solvated K+ and illite (a proxy for K-bearing clays) is +0.24‰, exceeding the current analytical precision, so equilibrium isotopic fractionation can induce measurable isotopic fractionations for this system at low temperature. These findings, however, cannot easily explain why the δ41K value of seawater is shifted by +0.6‰ relative to igneous rocks. -

New Occurrences from the Postmasburg Manganese Field, Northern Cape Province, South Africa
981 The Canadian Mineralogist Vol. 53, pp. 981-990 (2015) DOI: 10.3749/canmin.1500063 TOKYOITE, As-RICH TOKYOITE, AND NOÉLBENSONITE: NEW OCCURRENCES FROM THE POSTMASBURG MANGANESE FIELD, NORTHERN CAPE PROVINCE, SOUTH AFRICA GELU COSTIN Department of Geology, Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa Department of Earth Science, MS-126 Rice University, 6100 Main Street, Houston, Texas 77005, USA § BRENTON FAIREY AND HARILAOS TSIKOS Department of Geology, Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa ARNOLD GUCSIK Department of Geology, University of Johannesburg, Johannesburg, South Africa ABSTRACT Samples of manganese-rich rock containing two compositional varieties of tokyoite in association with noélbensonite were retrieved from a drill core obtained from the Postmasburg area in the Northern Cape Province of South Africa. The samples consist of fine-grained braunite, hematite, and hausmannite. Within this material abundant vugs are observed that are filled with witherite, baryte, and barytocalcite. In addition, As-rich tokyoite, tokyoite, and noélbensonite occur in the center of the vugs, in fine aggregates 0.1 to 1 mm in size. Individual As-rich tokyoite grains are typically 20–200 mm in size. The outer walls of the vugs are lined with microcrystalline K-feldspar, witherite, and/or sérandite. Textural evidence of the Ba-rich mineral phases in association with As-rich tokyoite suggest an epigenetic mode of formation of the observed assemblages, caused by V- and As-bearing alkali-rich fluids interacting with pre-existing Mn-rich minerals. Electron microprobe analysis (EMPA), electron backscatter diffraction analysis (EBSD), and Raman spectroscopy show that the As-rich tokyoite is a mineral belonging to the brackebuschite mineral group. -

Download the Scanned
INDEX TO VOLUME 24 Leading articles are in bold face type; notes, abstracts and reviews are in ordinary type. Only minerals for which definite data are siven are indexed. Abukumalite(ShinHata)....... 66 Brandenberger, E. Angewandte Adinolesof D.inasHead, Cornwall. Kristallstrukturlehre. IBook (Agrell). 63 review] 276 Agrell,S. O.. 63 Bray, J. M. Ilmenite-hematite- Alderman, A 277 magnetite relations in some Allen,V. T.. 194 emery ores. 162,183 Alurgite from California, new oc- Brochantite. (Palache,Richmond) 463 currenceof. (Webb). I z.t Brunckite. (Herzenberg) 350 Ammonium mica synthesized from Buerger,M. J. Crystal structure of vermiculite. (Gruner) 428 gudmundite.. 183 Anderson,B. W... 528 Bullard, F. M. Rosebudmeteorite, AngewandteKristallstrukturleh re. Milam Co., Texas 184,242 (Brandenberger)[Book review] 276 Burns,B. D. 528 Antlerite. (Palache). 293 Apatite, fluorescent,from Center Calcite,Fe-Mn. (Yosimura). 660 Strafford, N. Hamp. (Stewart) 274 Caledonite. (Palache, Richmond) 441 Australitesof unusualform. (Single- Canyon Diablo iron, identification ton). 63 of diamond in. (Ksanda, Awariute.(Owens, Burns). 528 Henderson).... 677 CesAro,G..... 280 "Baddelyite" from Alno-an error. Chao,S.H...... 277 (vonEckermann). 528 Chemicalanalyses of minerals,pres- Bannister,F. A. ....64,66 entationof. (Hey). 347 Barksdale, J. D. Silicified wood in Chlorite veins in serpentine near dolomite. .. ..181,699 Kings River, Calif. (Durrell, Bavenite,ne\\r occurrence of. (Clar- Macdonald). 452 ingbull). 277 Chrysoberyl occurrence near Behre,C. H., Jr. 181 Golden, Colo. (Waldschmidt, Beliankin,D. S.. 279 Gaines). 193,267 Bell,J. F. 181 Cinnabar,darkening of, in sunlight. Berman, H. Torsion microbalance (Dreyer).. +57 for determining specific grav- Claringbull,G. F.. 277,347 ity of minerals 182,434 Clay, Hawaiian ceramic, mineral -- and Gonyer, F, A. -

Green Aventurine Quartz: Mineralogical Characterization
Volume 20 No. 2 April 1986 GAGTL EDU Set 3 ^eJournal of Gemmology GEMMOLOGICAL ASSOCIATION OF GREAT BRITAIN OFFICERS AND COUNCIL President: Sir Frank Claringbull Ph.D., F.InsLP., F.G.S. Vice-Presidents: J. R. H. Chisholm, M.A., F.G .A. R. K. Mitchell, F.G.A. H. J. Wheeler, F.G.A. Chairman: D. J. Callaghan, F. G. A. Vice-Chairman: N. W. Deeks, F.G.A. Honorary Treasurer: N. B. Israel, F.G.A. Fellows elected to Council: C. R. Cavey B. Jackson M. J. O'Donoghue, L. F. Cole C. B. Jones M. A., F.G.S. R. W. Croydon D. M. Larcher P. G. Read, C.Eng., P. J. E. Daly, B.Sc. D. Morgan M.I.E.E., M.LE.R.E. A. J. French G. Neary A. W. R. Round S. E. Hiscox J. B. Nelson, Ph.D., F.R.M.S., E. Stern J. A. W. Hodgkinson F. InsLP., F.G.S. C. H. Winter D. Inkersole W. Nowak, C.Eng., F.R.Ac.S. Branch Chairmen: Midlands Branch: C. L. Hundy, F.G.A. North-West Branch: S. Hill, F.G.A. Sowh Yorkshire & Distria Branch: J. I. Reynolds, F.G.A. Examiners: E. A. Jobbins,B.Sc., F.I.M.M., F.G.A. A. J. Allnutt, M.Se.,Ph.D., F.G.A. R. R. Harding, B.Sc., D.Phil., F.G.A. G. H. Jones, B.Se., Ph.D., F.G.A. D. G. Kent, F.G.A. E. M. Bruton, F.G.A. P. Sadler, B.Sc., F.G.S., F.G.A.