Near-Infrared Spectroscopic Study of Chlorite Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mapping Phyllosilicates and Aqueous Alteration Products at Tyrrhena Terra, Mars
52nd Lunar and Planetary Science Conference 2021 (LPI Contrib. No. 2548) 1323.pdf MAPPING PHYLLOSILICATES AND AQUEOUS ALTERATION PRODUCTS AT TYRRHENA TERRA, MARS. F. H. Grant1 and J. L. Bishop2, 1University of California, Los Angeles (Los Angeles, CA; [email protected]), 2SETI Institute (Mountain View, CA). Summary: Outcrops of phyllosilicates and small sites of less common aqueous alteration minerals were mapped at Tyrrhena Terra on Mars using CRISM pa- rameters, and CRISM spectral data were collected to more closely analyze the mineralogy in this region. Fe- and Mg-rich smectite and chlorite group phyllosilicates are both detected at Tyrrhena Terra, including sapo- nite, chamosite, and clinochlore. Zeolites and car- bonates are among the rarer alteration minerals also observed at Tyrrhena Terra that further constrain the alteration history in this region. More chlorite than smectite is observed, indicating higher temperature alteration processes occurred at Tyrrhena Terra. Introduction: Tyrrhena Terra is a region in the southern hemisphere of Mars, between the Isidis and Hellas impact basins and south of Libya Montes. The selected study site (Fig. 1) is of particular interest be- cause it has a large concentration of phyllosilicate minerals exposed on the surface, among small outcrops of rarer aqueous alteration minerals [1]. Phyllosilicates are a crucial mineral group to study when striving to understand the aqueous alteration history of a region. Less common aqueous alteration minerals, including zeolite, carbonate, and hydrated sulfates, are also im- portant to further constrain the alteration history of a region [2]. For this research project, we focused pri- marily on identifying the phyllosilicate minerals smec- tite and chlorite. -

Metamorphic and Metasomatic Kyanite-Bearing Mineral
Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece) Alexandre Tarantola, Panagiotis Voudouris, Aurélien Eglinger, Christophe Scheffer, Kimberly Trebus, Marie Bitte, Benjamin Rondeau, Constantinos Mavrogonatos, Ian Graham, Marius Etienne, et al. To cite this version: Alexandre Tarantola, Panagiotis Voudouris, Aurélien Eglinger, Christophe Scheffer, Kimberly Tre- bus, et al.. Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece). Minerals, MDPI, 2019, 10.3390/min9040252. hal-02932247 HAL Id: hal-02932247 https://hal.archives-ouvertes.fr/hal-02932247 Submitted on 7 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. minerals Article Metamorphic and Metasomatic Kyanite-Bearing Mineral Assemblages of Thassos Island (Rhodope, Greece) Alexandre Tarantola 1,* , Panagiotis Voudouris 2 , Aurélien Eglinger 1, Christophe Scheffer 1,3, Kimberly Trebus 1, Marie Bitte 1, Benjamin Rondeau 4 , Constantinos Mavrogonatos 2 , Ian Graham 5, Marius Etienne 1 and Chantal Peiffert -

Alphabetical List
LIST L - MINERALS - ALPHABETICAL LIST Specific mineral Group name Specific mineral Group name acanthite sulfides asbolite oxides accessory minerals astrophyllite chain silicates actinolite clinoamphibole atacamite chlorides adamite arsenates augite clinopyroxene adularia alkali feldspar austinite arsenates aegirine clinopyroxene autunite phosphates aegirine-augite clinopyroxene awaruite alloys aenigmatite aenigmatite group axinite group sorosilicates aeschynite niobates azurite carbonates agate silica minerals babingtonite rhodonite group aikinite sulfides baddeleyite oxides akaganeite oxides barbosalite phosphates akermanite melilite group barite sulfates alabandite sulfides barium feldspar feldspar group alabaster barium silicates silicates albite plagioclase barylite sorosilicates alexandrite oxides bassanite sulfates allanite epidote group bastnaesite carbonates and fluorides alloclasite sulfides bavenite chain silicates allophane clay minerals bayerite oxides almandine garnet group beidellite clay minerals alpha quartz silica minerals beraunite phosphates alstonite carbonates berndtite sulfides altaite tellurides berryite sulfosalts alum sulfates berthierine serpentine group aluminum hydroxides oxides bertrandite sorosilicates aluminum oxides oxides beryl ring silicates alumohydrocalcite carbonates betafite niobates and tantalates alunite sulfates betekhtinite sulfides amazonite alkali feldspar beudantite arsenates and sulfates amber organic minerals bideauxite chlorides and fluorides amblygonite phosphates biotite mica group amethyst -

Medellín Dunite” Published Online 24 April 2020 Revisited: an Algebraic Approach and Proposal
Volume 2 Quaternary Chapter 2 Neogene https://doi.org/10.32685/pub.esp.36.2019.02 The Petrologic Nature of the “Medellín Dunite” Published online 24 April 2020 Revisited: An Algebraic Approach and Proposal of a New Definition of the Geological Body Paleogene Antonio GARCIA–CASCO1* , Jorge Julián RESTREPO2 , 1 [email protected] Universidad de Granada 3 4 Ana María CORREA–MARTÍNEZ , Idael Francisco BLANCO–QUINTERO , Departamento de Mineralogía y Petrología Avenida Fuente Nueva s/n, 18079 5 6 7 Cretaceous Joaquín Antonio PROENZA , Marion WEBER , and Lidia BUTJOSA Granada, España Instituto Andaluz de Ciencias de la Tierra, Abstract The “Medellín Dunite”, the main ultramafic body of the Central Cordillera of CSIC–UGR Avenida de las Palmeras, 4, 18100 Armilla Colombia, constitutes a fragment of oceanic lithospheric mantle formed at a back–arc Granada, España basin/incipient arc scenario emplaced onto the western continental margin of Pangaea 2 [email protected] Universidad Nacional de Colombia Jurassic during Triassic time. This body has been classically, and is still considered, mainly of Sede Medellín dunite composition. However, in spite of two subsequent metamorphic imprints that GEMMA Research Group Medellín, Colombiaa obscure the primary mantle mineralogical composition, there is petrographic and geo- 3 [email protected] chemical evidence that points to a harzburgitic nature of the unit. In order to overcome Universidade de Brasília Instituto de Geociências Triassic the petrographic effects of medium–T metamorphism, metasomatism, and serpentini- Servicio Geológico Colombiano zation, we analyzed published and new major–element geochemical data by means of Dirección de Geociencias Básicas Grupo de Estudios Geológicos Especiales algebraic methods to approximate the mantle mineralogical composition of ultramafic Calle 75 n.° 79A–51 Medellín, Colombia rocks. -

And Fe,Mg-Chlorite: a Genetic Linkage to W, (Cu, Mo) Mineralization in the Magmatic-Hydrothermal System at Borralha, Northern Portugal
Mineralogical Magazine, May 2018, Vol. 82(S1), pp. S259–S279 Fe-, Fe,Mn- and Fe,Mg-chlorite: a genetic linkage to W, (Cu, Mo) mineralization in the magmatic-hydrothermal system at Borralha, northern Portugal 1,* 1 2 I. BOBOS ,F.NORONHA AND A. MATEUS 1 Instituto de Ciências da Terra – Polo Porto, Departamento de Geociências, Ambiente e Ordenamento do Território, Faculdade de Ciências, Universidade do Porto, Rua do Campo Alegre 687, 4169-007 Porto, Portugal 2 Departamento de Geologia e IDL, Faculdade de Ciências, Universidade de Lisboa, C6, Campo Grande, 1746-016 Lisboa, Portugal [Received 23 January 2017; Accepted 22 December 2017; Associate Editor: Krister Sundblad] ABSTRACT A genetic linkage between W, (Cu, Mo)-mineralization and chlorite minerals, and the discrimination of different mineralization events in the magmatic-hydrothermal system of Borralha, northern Portugal, is discussed on the basis of textural relationships, crystal chemistry and stable isotopic data obtained from chlorite. Chlorite minerals were identified in assemblages with quartz, feldspars, tungstates and sulfides. X-ray diffraction studies of selected chlorite minerals shows a trioctahedral structural type. Electron probe micro-analyses identified four different compositions and associations: (1) Fe,Mn-chlorite with scheelite I; (2) Fe-chlorite with wolframite + scheelite II ± sulfide; (3) Fe,Mg-chlorite with molybdenite + bismuthinite; 3+ 2+ and (4) Mg,Fe-chlorite with chalcopyrite. The composition of Fe-chlorite (Al3.01Fe0.25Fe7.95Mn0.26 3+ 2+ Mg0.19)11.66(Si5.44Al2.56)8O20(OH)8 corresponds to daphnite and Fe,Mn-chlorite (Al2.69Fe0.02 Fe7.54 Mn1.08Mg0.62)11.89(Si5.31Al2.68)4O20(OH)8 to a mixed composition between daphnite and amesite. -

Ni-Co Laterites a Deposit Model
Ni-Co Laterites─A Deposit Model By Erin Marsh and Eric Anderson Open-File Report 2011–1259 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2011 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Suggested citation: Marsh, E.E. and Anderson, E.D., 2011, Ni-Co laterite deposits: U.S. Geological Survey Open-File Report 2011–1259, 9 p. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. ii Contents Introduction .................................................................................................................................................................... 1 History ........................................................................................................................................................................... 2 Profile Formation and Mineralogy ................................................................................................................................. -

Clinochlore (Mg,Fe2+)5Al(Si3al)O10(OH)8
2+ Clinochlore (Mg; Fe )5Al(Si3Al)O10(OH)8 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: In thin to thick pseudohexagonal crystals, with tapering pyramidal faces, to 10 cm. Commonly foliated, ¯brous, granular, earthy, massive. Twinning: Twin plane 001 ; twin axis [310], composition plane 001 . f g f g Physical Properties: Cleavage: 001 , perfect. Tenacity: Laminae °exible, inelastic. Hardness = 2{2.5 D(meas.) = 2.60{f3.02g D(calc.) = 2.628 Optical Properties: Transparent to translucent. Color: Grass-green, olive-green, yellowish, white; pink, rose-red; colorless to pale green or yellow in thin section. Streak: Greenish white to white. Luster: Pearly, greasy, dull. Optical Class: Biaxial (+) or ({). Pleochroism: Distinct; X = light yellow-green to light blue-green; Y = Z = light greenish yellow to light blue-green. Orientation: Y = b; Z c = 2±{9±. Dispersion: r < v: Absorption: Y Z > X or Z Y > Z. ® = 1.571{1.588 ¯ = 1.5^71{1.588 ' ' ° = 1.576{1.597 2V(meas.) = 0±{50± Cell Data: Space Group: C2=m: a = 5.350(3) b = 9.267(5) c = 14.27(1) ¯ = 96:35(5)± Z = 2 X-ray Powder Pattern: Synthetic (IIb structure); berthierine plus clinochlore easily mistaken for chamosite. 3.57 (100), 2.540 (100), 2.008 (100), 1.539 (100), 14.1 (80), 7.14 (80), 4.76 (80) Chemistry: (1) (2) (1) (2) SiO2 33.83 32.12 MgO 34.94 35.36 Al2O3 12.95 9.50 CaO 1.24 + Fe2O3 2.25 H2O 13.11 10.25 Cr2O3 7.88 H2O¡ 2.04 FeO 3.02 1.98 Total 100.10 100.37 2+ 3+ (1) Zillertal, Austria; corresponds to (Mg4:94Fe0:24)§=5:18(Al0:65Fe0:16)§=0:81 (Si3:21Al0:79)§=4:00O10(OH)8: (2) Deer Park, Wyoming, USA; corresponds to 2+ 3+ (Mg5:05Fe0:16Ca0:13)§=5:34(Cr0:60Al0:15Fe0:15)§=0:90(Si3:08Al0:92)§=4:00O10(OH)8: Polymorphism & Series: Forms a series with chamosite; stacking disorder is common. -

Phyllosilicates 1 Mica Minerals Etc
UNIVERSITY OF SOUTH ALABAMA GY 302: Crystallography & Mineralogy Lecture 23: Phyllosilicates 1 Mica Minerals etc. Last Time Class VIII Minerals: Inosilicates (part 2) 1. Amphiboles Inosilicates (double chain) Two major sub groups of amphiboles are distinguished on the basis of crystal structure: 1) Orthorhombic Amphiboles 2) Monoclinic Amphiboles http://www.uwgb.edu/dutchs/GRAPHIC0/ROCKMIN/ATOM-STRUCT/amphibchain.gif Inosilicates (double chain) General formula: XY((Al,Si)4O11)2(OH)2 2+ + X[larger ions] = Ca , Na 2+ 3+ 2+/3+ 2+/3+ Y[smaller ions] = Mg , Al , Fe , Mn ) http://www.tulane.edu/~sanelson/images/amphibole_cleavage.gif Inosilicates (double chain) We recognize several amphibole series (solid solution) 1) Anthophyllite Series (Orthorhombic) 2) Cummingtonite Series (Monoclinic) 3) Actinolite Series (Monoclinic) 4) Hornblende Series (Monoclinic) 5) Glaucophane Series (Monoclinic) 6) Riebeckite Series (Monoclinic) 7) Arfvedsonite-Eckermannite Series (Mono) http://www.tulane.edu/~sanelson/images/amphibole-compositions.gif “Hornblende”: (Ca,Na,K)2-3(Mg,Fe,Al)5Si6(Si,Al)2O22(OH)2 “Glaucophane”: Na2Mg3Al2Si8O22(OH)2 2+ 3+ “Riebeckite”: Na2Fe 3Fe 2Si8O22(OH)2 2+ “Arvedsonite”: Na3(Mg,Fe )4AlSi8O22(OH)2 Asbestos Minerals Several minerals with a fibrous habit that have useful economic properties (flexible, high melting points, tensile strength, heat resistance) Amphibole Asbestos minerals: Anthophyllite, Tremolite, Amosite, Actinolite, Crocidolite Serpentine Asbestos minerals: Clinochrysotile, Orthochrysotile Crocidolite (“blue asbestos”) is part of the Riebeckite series and is the worst of the asbestos minerals (at least according to legal websites) http://www.earthsci.unimelb.edu.au/Thomas/lteng/engeimg/enge0247a.JPG Today’s Agenda Phyllosilicates Part 1 1. General chemistry/structure 2. -
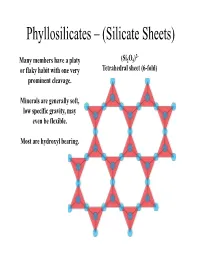
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups. -

Petrology and Geochemistry of Selected Talc-Bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont
Petrology and Geochemistry of Selected Talc-bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont GEOLOGICAL SURVEY PROFESSIONAL PAPER 345 Petrology and Geochemistry of Selected Talc-bearing Ultramafic Rocks and Adjacent Country Rocks in North-Central Vermont By ALFRED H. CHIDESTER GEOLOGICAL SURVEY PROFESSIONAL PAPER 345 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1962 UNITED STATES DEPARTMENT OF THE INTERIOR STEW ART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publication as follows : Chidester, Alfred Herman, 1914- Petrology and geochemistry of selected talc bearing- ultra- mafic rocks and adjacent country rocks in north-central Vermont. Washington, U.S. Govt. Print. Off., 1961. vii, 207 p. illus., maps (7 fold. col. in pocket) cliagrs.. tables. 29 cm. (U.S. Geological Survey. Professional paper 345) Bibliography: p. 205-207. 1. Petrology Vermont. 2. Talc Vermont. 3. Mines and mineral resources Vermont. 4. Geochemistry Vermont. 5. Rocks, Igne ous Vermont. I. Title. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, B.C. CONTENTS Page Geology of the Barnes Hill, Waterbury mine, and Mad Abstract. __________________________________________ 1 River localities Continued Introduction. ______________________________________ 3 Petrography Continued Location and history. ___________________________ 3 Schist Continued Barnes Hill locality _________________________ 3 Mineralogy, etc. Continued Page Waterbury mine locality_____--_---__________ 4 . Ilmenite, rutile, and sphene_ _________ 52 Mad River locality_________---_----__-______ 4 Garnet.___________________________ 52 Previous investigations __________________________ 5 Apatite__. _________________________ 53 Fieldwork and acknowledgments. -___-_---________ 5 Epidote and allanite _______________ 53 Geologic setting. ___________________________________ 6 Other minerals _____________________ 53 Regional setting. -

Phyllosilicates (Micas, Chlorite, Talc, & Serpentine)
Phyllosilicates EENS 211 Mineralogy Tulane University Prof. Stephen A. Nelson Phyllosilicates (Micas, Chlorite, Talc, & Serpentine) This document last updated on 12-Nov-2008 Phyllosilicates (Sheet Silicates) The phyllosilicates, or sheet silicates, are an important group of minerals that includes the micas, chlorite, serpentine, talc, and the clay minerals. Because of the special importance of the clay minerals as one of the primary products of chemical weathering and one of the more abundant constituents of sedimentary rocks, they will be discussed in more detail in the next lecture. The basic structure of the phyllosilicates is based on -4 interconnected six member rings of SiO4 tetrahedra that extend outward in infinite sheets. Three out of the 4 oxygens from each tetrahedra are shared with other -2 tetrahedra. This leads to a basic structural unit of Si2O5 . Most phyllosilicates contain hydroxyl ion, OH-, with the OH located at the center of the 6 membered rings, as shown here. -3 Thus, the group becomes Si2O5(OH) . When other cations are bonded to the SiO4 sheets, they share the apical oxygens and the (OH) ions which bond to the other cations in octahedral coordination. This forms a layer of cations, usually Fe+2, Mg+2, or Al+3, that occur in octahedral coordination with the O and OH ions of the tetrahedral layer. As shown, here, the triangles become the faces of the octahedral groups that can bind to the tetrahedral layers. The octahedral layers take on the structure of either Brucite [Mg(OH)3], if the cations are +2 ions like Mg+2 or Fe+2, or Gibbsite +3 [Al(OH)3], if the cations are +3 like Al .