ATOMIC WEIGHTS of the ELEMENTS 1999 (IUPAC Technical Report)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment Outcome Following Use of the Erbium, Chromium:Yttrium
Treatment outcome following use IN BRIEF • Highlights that successful treatment RESEARCH of peri-implantitis is unpredictable and of the erbium, chromium:yttrium, usually involves the need for surgery. • Outlines a minimalistic treatment approach for peri-implantitis with promising results after six months. scandium, gallium, garnet laser • Suggests that the protocol described may be of use in a pilot study or controlled in the non-surgical management clinical trial. of peri-implantitis: a case series R. Al-Falaki,*1 M. Cronshaw2 and F. J. Hughes3 VERIFIABLE CPD PAPER Aim To date there is no consensus on the appropriate usage of lasers in the management of peri-implantitis. Our aim was to conduct a retrospective clinical analysis of a case series of implants treated using an erbium, chromium:yttrium, scandium, gallium, garnet laser. Materials and methods Twenty-eight implants with peri-implantitis in 11 patients were treated with an Er,Cr:YSGG laser (68 sites >4 mm), using a 14 mm, 500 μm diameter, 60º (85%) radial firing tip (1.5 W, 30 Hz, short (140 μs) pulse, 50 mJ/pulse, 50% water, 40% air). Probing depths were recorded at baseline after 2 months and 6 months, along with the presence of bleeding on probing. Results The age range was 27-69 years (mean 55.9); mean pocket depth at baseline was 6.64 ± SD 1.48 mm (range 5-12 mm),with a mean residual depth of 3.29 ± 1.02 mm (range 1-6 mm) after 2 months, and 2.97 ± 0.7 mm (range 1-9 mm) at 6 months. -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Package 'Ciaawconsensus'
Package ‘CIAAWconsensus’ September 19, 2018 Type Package Title Isotope Ratio Meta-Analysis Version 1.3 Author Juris Meija and Antonio Possolo Maintainer Juris Meija <[email protected]> Description Calculation of consensus values for atomic weights, isotope amount ratios, and iso- topic abundances with the associated uncertainties using multivariate meta-regression ap- proach for consensus building. License Unlimited LazyData yes Imports mvtnorm, stringr, numDeriv, stats, Matrix NeedsCompilation no Repository CRAN Date/Publication 2018-09-19 13:30:12 UTC R topics documented: abundances2ratios . .2 at.weight . .3 ciaaw.mass.2003 . .4 ciaaw.mass.2012 . .5 ciaaw.mass.2016 . .6 iridium.data . .6 mmm ............................................7 normalize.ratios . .8 platinum.data . .9 Index 10 1 2 abundances2ratios abundances2ratios Isotope ratios of a chemical element from isotopic abundances Description This function calculates the isotope ratios of a chemical element from the given isotopic abundances and their uncertainties. The uncertainty evaluation is done using the propagation of uncertainty and the missing correlations between the isotopic abundances are reconstructed using Monte Carlo methods. Usage abundances2ratios(x, ux, ref=1, iterations=1e4) Arguments x A vector of isotopic abundances of an element ux Standard uncertainties of x ref Index to specify the desired reference isotope for isotope amount ratios iterations Number of iterations for isotopic abundance correlation mapping Details Situations are often encountered where isotopic abundances are reported but not the isotope ratios. In such cases we reconstruct the isotope ratios that are consistent with the abundances and their uncertainties. Given only the abundances and their uncertainties, for elements with four or more isotopes one cannot unambiguously infer the uncertainties of the ratios due to the unknown correla- tions between isotopic abundances. -
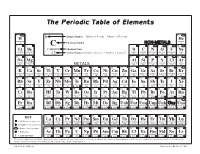
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

Chalcogenide-Bound Erbium Complexes: Paradigm Molecules for Infrared Fluorescence Emission
5130 Chem. Mater. 2005, 17, 5130-5135 Chalcogenide-Bound Erbium Complexes: Paradigm Molecules for Infrared Fluorescence Emission G. A. Kumar and Richard E. Riman* Department of Ceramic & Material Engineering, The State UniVersity of New Jersey, 607 Taylor Road, Piscataway, New Jersey 08854-8065 L. A. Diaz Torres and O. Barbosa Garcia Centro de InVestigaciones en Optica, Leon Gto., Mexico City 37150, Mexico Santanu Banerjee, Anna Kornienko, and John G. Brennan Department of Chemistry and Chemical Biology, Rutgers, The State UniVersity of New Jersey, 607 Taylor Road, Piscataway, New Jersey 08854-8087 ReceiVed April 11, 2005. ReVised Manuscript ReceiVed June 23, 2005 The near-infrared luminescence properties of the nanoscale erbium ceramic cluster (THF)14Er10S6- Se12I6 (Er10, where THF ) tetrahydrofuran) and the molecular erbium thiolate (DME)2Er(SC6F5)3 (Er1, where DME ) 1,2-dimethoxyethane) were studied by optical absorption, photoluminescence, and 4 4 vibrational spectroscopy. The calculated radiative decay time of 4 ms for the I13/2 f I15/2 transition is comparable to the reported values for previously reported organic complexes. The recorded emission 4 4 spectrum of the I13/2 f I15/2 transition was centered at 1544 nm with a bandwidth of 61 and 104 nm for Er10 and Er1, respectively, with stimulated emission cross sections of 1.3 × 10-20 cm2 (Er10) and 0.8 × 10-20 cm2 (Er1) that are comparable to those of solid-state inorganic systems. Lifetime measurements of the 1544 nm decay showed a fluorescence decay time of the order of 3 ms for Er10 that, together with the radiative decay time, yielded a quantum efficiency above 78%, which is considered to be the highest reported value for a “molecular” erbium compound. -
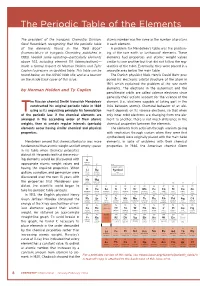
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

Erbium Doped Silicon As an Optoelectronic Semiconductor Material by Yong-Gang Frank Ren B.S
? Erbium Doped Silicon as an Optoelectronic Semiconductor Material by Yong-Gang Frank Ren B.S. Materials Science, Beijing University of Aeronautics, 1985 M.S. Physics, University of Nebraska-Lincoln, 1989 MBA, MIT Sloan School of Management, 1994 Submitted to the Department of Materials Science and Engineering in partial fulfillment of the requirements for the degree of DOCTOR of PHILOSOPHY in Electronic Materials at the Massachusetts Institute of Technology May 1994 © Massachusetts Institute of Technology 1994. All rights reserved. Signature of Author ...... .... ...... '..V. Department of Materials Science and Engineering April 29, 1994 Certified by..' 1 Lionel C. Kimerling Professor Thesis Supervisor Acceptedby... Carl V. Thompson II Professor of Electronic Materials Chair, Departmental Committee on Graduate Students A '1,"' .. .1.. '4 AU 18 1994 iSence Erbium Doped Silicon as an Optoelectronic Semiconductor Material by Yong-Gang Frank Ren Submitted to the Department of Materials Science and Engineering on April 29, 1994, in partial fulfillment of the requirements for the degree of DOCTOR of PHILOSOPHY in Electronic Materials Abstract In this thesis, the materials aspects of erbium-doped silicon (Si:Er) are studied to maximize Si:Er luminescence intensity and to improve Si:Er performance as an opto- electronic semiconductor material. Studies of erbium (Er) and silicon (Si) reactivity, erbium diffusion and solubility in silicon, Si:Er heat treatment processing with oxy- gen and fluorine co-implantation and the mechanism of Si:Er light emission have been carried out to define optimum processing conditions and to understand the nature of optically active centers in Si:Er. In the erbium and silicon reactivity studies, a ternary phase diagram of Er-Si-O was determined.