A Frog's Eye View
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Character Assessment, Genus Level Boundaries, and Phylogenetic Analyses of the Family Rhacophoridae: a Review and Present Day Status
Contemporary Herpetology ISSN 1094-2246 2000 Number 2 7 April 2000 CHARACTER ASSESSMENT, GENUS LEVEL BOUNDARIES, AND PHYLOGENETIC ANALYSES OF THE FAMILY RHACOPHORIDAE: A REVIEW AND PRESENT DAY STATUS Jeffery A. Wilkinson ([email protected]) and Robert C. Drewes ([email protected]) Department of Herpetology, California Academy of Sciences, Golden Gate Park, San Francisco, California 94118 Abstract. The first comprehensive phylogenetic analysis of the family Rhacophoridae was conducted by Liem (1970) scoring 81 species for 36 morphological characters. Channing (1989), in a reanalysis of Liem’s study, produced a phylogenetic hypothesis different from that of Liem. We compared the two studies and produced a third phylogenetic hypothesis based on the same characters. We also present the synapomorphic characters from Liem that define the major clades and each genus within the family. Finally, we summarize intergeneric relationships within the family as hypothesized by other studies, and the family’s current status as it relates to other ranoid families. The family Rhacophoridae is comprised of over 200 species of Asian and African tree frogs that have been categorized into 10 genera and two subfamilies (Buergerinae and Rhacophorinae; Duellman, 1993). Buergerinae is a monotypic category that accommodates the relatively small genus Buergeria. The remaining genera, Aglyptodactylus, Boophis, Chirixalus, Chiromantis, Nyctixalus, Philautus, Polyp edates, Rhacophorus, and Theloderma, comprise Rhacophorinae (Channing, 1989). The family is part of the neobatrachian clade Ranoidea, which also includes the families Ranidae, Hyperoliidae, Dendrobatidae, Arthroleptidae, the genus Hemisus, and possibly the family Microhylidae. The Ranoidea clade is distinguished from other neobatrachians by the synapomorphic characters of completely fused epicoracoid cartilages, the medial end of the coracoid being wider than the lateral end, and the insertion of the semitendinosus tendon being dorsal to the m. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -
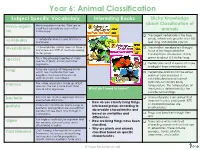
Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge About Classification of Micro-Organi Micro-Organisms Are Tiny
Year 6: Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge about Classification of micro-organi Micro-organisms are tiny. They are so small they can only be seen with a animals sm microscope. ❑ The largest vertebrate is the blue A vertebrate animal is one that has a whale, which can grow to over 100 vertebrates backbone. feet long and 400,000 pounds. An Invertebrate animal does not have a invertebrates ❑ The smallest vertebrate is thought backbone and 97% of creatures belong to be a tiny frog called the to this group. Paedophryne amauensis. It only This is the grouping together of similar grows to about 0.3 inches long. species species of plant, animal and other organisms. ❑ Vertebrates tend to be much more intelligent than invertebrates. Fungi are a group of living organisms fungi which are classified in their own ❑ Vertebrate animals can be either kingdom. This means they are not warm or cold-blooded. A animals, plants, or bacteria. cold-blooded animal cannot The whole organism is made up of just maintain a constant body monera one cell. The cell is more basic than temperature. The temperature of cells of other organisms. What do I need to know? their body is determined by the outside surroundings. Bacteria are tiny little organisms that are An invertebrate is an animal that bacteria ❑ everywhere around us. • How do you classify living things does not have a backbone. 97% Protists are not animals, plants, fungi, or of all animal species are protista into broad groups according to bacteria. Many protists are so small that invertebrates. -

Summary Conservation Action Plans for Mongolian Reptiles and Amphibians
Summary Conservation Action Plans for Mongolian Reptiles and Amphibians Compiled by Terbish, Kh., Munkhbayar, Kh., Clark, E.L., Munkhbat, J. and Monks, E.M. Edited by Munkhbaatar, M., Baillie, J.E.M., Borkin, L., Batsaikhan, N., Samiya, R. and Semenov, D.V. ERSITY O IV F N E U D U E T C A A T T S I O E N H T M ONGOLIA THE WORLD BANK i ii This publication has been funded by the World Bank’s Netherlands-Mongolia Trust Fund for Environmental Reform. The fi ndings, interpretations, and conclusions expressed herein are those of the author(s) and do not necessarily refl ect the views of the Executive Directors of the International Bank for Reconstruction and Development / the World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colours, denominations, and other information shown on any map in this work do not imply any judgement on the part of the World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. The World Conservation Union (IUCN) have contributed to the production of the Summary Conservation Action Plans for Mongolian Reptiles and Amphibians, providing technical support, staff time, and data. IUCN supports the production of the Summary Conservation Action Plans for Mongolian Reptiles and Amphibians, but the information contained in this document does not necessarily represent the views of IUCN. Published by: Zoological Society of London, Regent’s Park, London, NW1 4RY Copyright: © Zoological Society of London and contributors 2006. -

Phylogeography Reveals an Ancient Cryptic Radiation in East-Asian Tree
Dufresnes et al. BMC Evolutionary Biology (2016) 16:253 DOI 10.1186/s12862-016-0814-x RESEARCH ARTICLE Open Access Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages Christophe Dufresnes1, Spartak N. Litvinchuk2, Amaël Borzée3,4, Yikweon Jang4, Jia-Tang Li5, Ikuo Miura6, Nicolas Perrin1 and Matthias Stöck7,8* Abstract Background: In contrast to the Western Palearctic and Nearctic biogeographic regions, the phylogeography of Eastern-Palearctic terrestrial vertebrates has received relatively little attention. In East Asia, tectonic events, along with Pleistocene climatic conditions, likely affected species distribution and diversity, especially through their impact on sea levels and the consequent opening and closing of land-bridges between Eurasia and the Japanese Archipelago. To better understand these effects, we sequenced mitochondrial and nuclear markers to determine phylogeographic patterns in East-Asian tree frogs, with a particular focus on the widespread H. japonica. Results: We document several cryptic lineages within the currently recognized H. japonica populations, including two main clades of Late Miocene divergence (~5 Mya). One occurs on the northeastern Japanese Archipelago (Honshu and Hokkaido) and the Russian Far-East islands (Kunashir and Sakhalin), and the second one inhabits the remaining range, comprising southwestern Japan, the Korean Peninsula, Transiberian China, Russia and Mongolia. Each clade further features strong allopatric Plio-Pleistocene subdivisions (~2-3 Mya), especially among continental and southwestern Japanese tree frog populations. Combined with paleo-climate-based distribution models, the molecular data allowed the identification of Pleistocene glacial refugia and continental routes of postglacial recolonization. Phylogenetic reconstructions further supported genetic homogeneity between the Korean H. -

High Abundance of Invasive African Clawed Frog Xenopus Laevis in Chile: Challenges for Their Control and Updated Invasive Distribution
Management of Biological Invasions (2019) Volume 10, Issue 2: 377–388 CORRECTED PROOF Research Article High abundance of invasive African clawed frog Xenopus laevis in Chile: challenges for their control and updated invasive distribution Marta Mora1, Daniel J. Pons2,3, Alexandra Peñafiel-Ricaurte2, Mario Alvarado-Rybak2, Saulo Lebuy2 and Claudio Soto-Azat2,* 1Vida Nativa NGO, Santiago, Chile 2Centro de Investigación para la Sustentabilidad & Programa de Doctorado en Medicina de la Conservación, Facultad de Ciencias de la Vida, Universidad Andres Bello, Republica 440, Santiago, Chile 3Facultad de Ciencias Exactas, Departamento de Matemáticas, Universidad Andres Bello, Santiago, Republica 470, Santiago, Chile Author e-mails: [email protected] (CSA), [email protected] (MM), [email protected] (DJP), [email protected] (APR), [email protected] (MAR), [email protected] (SL) *Corresponding author Citation: Mora M, Pons DJ, Peñafiel- Ricaurte A, Alvarado-Rybak M, Lebuy S, Abstract Soto-Azat C (2019) High abundance of invasive African clawed frog Xenopus Invasive African clawed frog Xenopus laevis (Daudin, 1802) are considered a major laevis in Chile: challenges for their control threat to aquatic environments. Beginning in the early 1970s, invasive populations and updated invasive distribution. have now been established throughout much of central Chile. Between September Management of Biological Invasions 10(2): and December 2015, we studied a population of X. laevis from a small pond in 377–388, https://doi.org/10.3391/mbi.2019. 10.2.11 Viña del Mar, where we estimated the population size and evaluated the use of hand nets as a method of control. First, by means of a non-linear extrapolation Received: 26 October 2018 model using the data from a single capture session of 200 min, a population size of Accepted: 7 March 2019 1,182 post-metamorphic frogs (range: 1,168–1,195 [quadratic error]) and a density Published: 16 May 2019 of 13.7 frogs/m2 (range: 13.6–13.9) of surface water were estimated. -

Anura: Calyptocephalellidae)
Phyllomedusa 19(1):99–106, 2020 © 2020 Universidade de São Paulo - ESALQ ISSN 1519-1397 (print) / ISSN 2316-9079 (online) doi: http://dx.doi.org/10.11606/issn.2316-9079.v19i1p99-106 Chondrocranial and hyobranchial structure in two South American suctorial tadpoles of the genus Telmatobufo (Anura: Calyptocephalellidae) J. Ramón Formas1 and César C. Cuevas1,2 ¹ Laboratorio de Sistemática, Instituto de Ciencias Marinas y Limnológicas, Universidad Austral de Chile. Valdivia, Chile. E-mail: [email protected]. ² Departamento de Ciencias Biológicas y Químicas, Universidad Católica de Temuco. Chile. E-mail: [email protected]. Abstract Chondrocranial and hyobranchial structure in two South American suctorial tadpoles of the genus Telmatobufo (Anura: Calyptocephalellidae). The chondrocranium, hyobranchium, rectus abdominis muscle, and epaxial musculature of Telmatobufo australis and T. ignotus are described. In addition, these structures were compared wih those of the non-suctorial Calyptocephalella gayi, the sister group of Telmatobufo. Keywords: Evolution, larval morphology, southern Chile, suctorial tadpoles. Resumen Estructura del condrocráneo y aparato hiobranquial de dos renacuajos suctores sudamericanos del género Telmatobufo (Anura: Calyptocephallidae). Se describen los condrocráneos, aparatos hiobranquiales, músculo recto abdominal, y la musculatura epaxial de Telmabufo australis y T. ignotus. En adición, los renacuajos de Telmatobufo se comparan con los de Calyptocephalella gayi, el grupo hermano de Telmatobufo. Palabras claves: evolución, morfología larvaria, renacuajos suctores, sur de Chile. Resumo Estrutura do condrocrânio e do aparelho hiobranquial de dois girinos suctoriais sulamericanos do gênero Telmatobufo (Anura: Calyptocephalellidae). Descrevemos aqui o condrocrânio, o aparelho hiobranquial, o músculo reto-abdominal e a musculatura epiaxial de Telmabufo australis e T. ignotus. Além disso, comparamos os girinos de Telmatobufo aos de Calyptocephalella gayi, o grupo-irmão de Telmatobufo. -

GAYANA Evidence of Predation on the Helmeted Water
GAYANA Gayana (2020) vol. 84, No. 1, 64-67 DOI: XXXXX/XXXXXXXXXXXXXXXXX SHORT COMMUNICATION Evidence of predation on the Helmeted water toad Calyptocephalella gayi (Duméril & Bibron, 1841) by the invasive African clawed frog Xenopus laevis (Daudin 1802) Evidencia de depredación sobre la rana chilena Calyptocephalella gayi (Duméril & Bibron, 1841) por la rana africana invasora Xenopus laevis (Daudin 1802) Pablo Fibla1,*, José M. Serrano1,2, Franco Cruz-Jofré1,3, Alejandra A. Fabres1, Francisco Ramírez4, Paola A. Sáez1, Katherin E. Otálora1 & Marco A. Méndez1 1Laboratorio de Genética y Evolución, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Santiago, Chile. 2Laboratorio de Comunicación Animal, Vicerrectoría de Investigación y Postgrado, Universidad Católica del Maule, Talca, Chile. 3Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Viña del Mar, Chile. 4Instituto de Geografía, Pontificia Universidad Católica de Chile, Santiago, Chile. *E-mail: [email protected] ABSTRACT We report the first record of predation on a Helmeted water toad Calyptocephalella gayi tadpole by an adult specimen of the invasive African clawed frog Xenopus laevis in the locality of Pichi, Alhué (Santiago Metropolitan Region). This finding is discussed in the light of new sightings, in which both species have been detected to coexist in different localities of central Chile. Keywords: Anura, Central Chile, invasive species, semi-urban wetlands RESUMEN Se reporta el primer registro de depredación de una larva de rana chilena Calyptocephalella gayi por un espécimen adulto de la rana africana invasora Xenopus laevis en la localidad de Pichi, Alhué (Región Metropolitana de Santiago). Este hallazgo es discutido a la luz de nuevos avistamientos en los cuales ambas especies han sido detectadas coexistiendo en diferentes localidades de Chile central. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Bullock's False Toad, Telmatobufo Bullocki
CONSERVATION 583 CONSERVATION Herpetological Review, 2013, 44(4), 583–590. © 2013 by Society for the Study of Amphibians and Reptiles Status and Conservation of a Gondwana Legacy: Bullock’s False Toad, Telmatobufo bullocki (Amphibia: Anura: Calyptocephalellidae) Lowland temperate forests often suffer from anthropo- genus contains two (possibly three) other species: T. australis genic influences owing to their productive soils and ease of (Formas 1972), T. bullocki (Schmidt 1952), and, questionably, accessibility (Pérez et al. 2009). In fact, extensive alteration of Chile’s lowland temperate forests has occurred for over four DANTÉ B. FENOLIO* centuries (Armesto et al. 1994; Donoso and Lara 1996; Pérez Department of Conservation Research, Atlanta Botanical Garden, 1345 et al. 2009). The fragmented forests that remain in Chile are Piedmont Rd. NE, Atlanta, Georgia 30309, USA sandwiched between the Andes Mountains to the east, the Pa- VIRGINIA MORENO-PUIG cific Ocean to the west, and the Atacama Desert to the north. A Ecology, Conservation & Behavior Group, Institute of Natural and narrow strip of southern Chile and adjacent Argentina house Mathematical Sciences, Massey University, Auckland, New Zealand all that remains of the temperate humid forests of the region e-mail: [email protected] (Aravena et al. 2002). These forests are biologically unique MICHAEL G. LEVY Department of Population Health and Pathobiology, North Carolina owing to isolation since the Tertiary Period and they host sig- State University College of Veterinary Medicine, 4700 Hillsborough Street, nificant numbers of endemic plants and animals (Aravena et Raleigh, North Carolina 27606, USA al. 2002; Armesto et al. 1996; Arroyo et al. 1996; Villagrán and e-mail: [email protected] Hinojosa 1997). -

F3999f15-C572-46Ad-Bbbe
THE STATUTES OF THE REPUBLIC OF SINGAPORE ENDANGERED SPECIES (IMPORT AND EXPORT) ACT (CHAPTER 92A) (Original Enactment: Act 5 of 2006) REVISED EDITION 2008 (1st January 2008) Prepared and Published by THE LAW REVISION COMMISSION UNDER THE AUTHORITY OF THE REVISED EDITION OF THE LAWS ACT (CHAPTER 275) Informal Consolidation – version in force from 22/6/2021 CHAPTER 92A 2008 Ed. Endangered Species (Import and Export) Act ARRANGEMENT OF SECTIONS PART I PRELIMINARY Section 1. Short title 2. Interpretation 3. Appointment of Director-General and authorised officers PART II CONTROL OF IMPORT, EXPORT, ETC., OF SCHEDULED SPECIES 4. Restriction on import, export, etc., of scheduled species 5. Control of scheduled species in transit 6. Defence to offence under section 4 or 5 7. Issue of permit 8. Cancellation of permit PART III ENFORCEMENT POWERS AND PROCEEDINGS 9. Power of inspection 10. Power to investigate and require information 11. Power of entry, search and seizure 12. Powers ancillary to inspections and searches 13. Power to require scheduled species to be marked, etc. 14. Power of arrest 15. Forfeiture 16. Obstruction 17. Penalty for false declarations, etc. 18. General penalty 19. Abetment of offences 20. Offences by bodies corporate, etc. 1 Informal Consolidation – version in force from 22/6/2021 Endangered Species (Import and 2008 Ed. Export) CAP. 92A 2 PART IV MISCELLANEOUS Section 21. Advisory Committee 22. Fees, etc., payable to Board 23. Board not liable for damage caused to goods or property as result of search, etc. 24. Jurisdiction of court, etc. 25. Composition of offences 26. Exemption 27. Service of documents 28. -

Rampant Tooth Loss Across 200 Million Years of Frog Evolution
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Rampant tooth loss across 200 million years of frog evolution 2 3 4 Daniel J. Paluh1,2, Karina Riddell1, Catherine M. Early1,3, Maggie M. Hantak1, Gregory F.M. 5 Jongsma1,2, Rachel M. Keeffe1,2, Fernanda Magalhães Silva1,4, Stuart V. Nielsen1, María Camila 6 Vallejo-Pareja1,2, Edward L. Stanley1, David C. Blackburn1 7 8 1Department of Natural History, Florida Museum of Natural History, University of Florida, 9 Gainesville, Florida USA 32611 10 2Department of Biology, University of Florida, Gainesville, Florida USA 32611 11 3Biology Department, Science Museum of Minnesota, Saint Paul, Minnesota USA 55102 12 4Programa de Pós Graduação em Zoologia, Universidade Federal do Pará/Museu Paraense 13 Emilio Goeldi, Belém, Pará Brazil 14 15 *Corresponding author: Daniel J. Paluh, [email protected], +1 814-602-3764 16 17 Key words: Anura; teeth; edentulism; toothlessness; trait lability; comparative methods 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license.