Rampant Tooth Loss Across 200 Million Years of Frog Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Analysis of Signal Peptides in Amphibian Antimicrobial Secretions
Journal of Genetics, Vol. 97, No. 5, December 2018, pp. 1205–1212 © Indian Academy of Sciences https://doi.org/10.1007/s12041-018-1018-5 RESEARCH ARTICLE Genetic analysis of signal peptides in amphibian antimicrobial secretions L. O. PÉREZ1, N. L. CANCELARICH2, S. AGUILAR2,3,N.G.BASSO4 and M. M. MARANI2∗ 1Instituto Patagónico de Ciencias Sociales y Humanas (IPCSH), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Puerto Madryn, Argentina 2Instituto Patagónico para el Estudio de Ecosistemas Continentales (IPEEC),Puerto Madryn, Argentina 3Facultad de Ciencias Naturales, Sede Puerto Madryn, Universidad Nacional de la Patagonia San Juan Bosco, 3051 Brown Boulevard, Puerto Madryn, Argentina 4Instituto de Diversidad y Evolución Austral (IDEAus), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), 2915 Brown Boulevard, Puerto Madryn, Argentina *For correspondence. E-mail: [email protected]. Received 22 February 2018; accepted 1 May 2018; published online 7 November 2018 Abstract. Amphibian secretion is an important source of bioactive molecules that naturally protect the skin against noxious microorganisms. Collectively called antimicrobial peptides (AMPs), these molecules have a wide spectrum of action, targeting viruses, bacteria and fungi. Like many membrane and secreted proteins, AMPs have cleavable signal sequences that mediate and translocate the nascent polypeptide chains into the endoplasmic reticulum. Although it is accepted that the signal peptides (SPs) are simple and interchangeable, there is neither sequence nor structure that is conserved among all gene families. They derived from a common ancestor but developed different traits as they adapt to distinct environmental pressures. The aim of this study was to provide an overview of the diversity of SPs of the frog, taking into account reported cDNA sequences and the evolutionary relationship among them. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

A New Species of the Genus Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) from Eastern India with Notes on Indian Species
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.07.455509; this version posted August 9, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. A new species of the genus Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) from eastern India with notes on Indian species Somnath Bhakat1 and Soumendranath Bhakat2 1Dept. of Zoology, Rampurhat College, Rampurhat- 731224, Dist. Birbhum, W. b. [email protected] ORCID: 0000-0002-4926-2496 2 Vivekanandapally, P. O. Suri- 731101, Dist. Birbhum, W. B. India, Formerly (Biophysical Chemistry Lab., Lund University, Sweden. E-mail: [email protected] ORCID: 0000-0002-1184-9259 Abstract A new species of the genus Microhyla, Microhyla bengalensis sp. nov., described from West Bengal state, India. The new species is distinguished from its congeners by a combination of the following morphological characters: 1) Small in size (SVL= 16.2 mm. in male); 2) truncated snout in dorsal view; 3) head wider than long (HW: HL= 1.36); 4) canthus rostralis and tympanum are indistinct; 5) nostril placed on the dorsal side of the snout; 6) tibiotarsal articulation not reaching the eye; 7) fingers and toes without disc; 8) toe webbing basal; 9) thigh and foot length are equal and smaller than shank; 10) skin tuberculated on dorsum; 11) ‘teddy bear’ dark brown mark on dorsum; 12) an inverted ‘V’-shaped dark brown mark above the vent. -

Ecol 483/583 – Herpetology Lab 3: Amphibian Diversity 2: Anura Spring 2010
Ecol 483/583 – Herpetology Lab 3: Amphibian Diversity 2: Anura Spring 2010 P.J. Bergmann & S. Foldi (Modified from Bonine & Foldi 2008) Lab objectives The objectives of today’s lab are to: 1. Familiarize yourself with Anuran diversity. 2. Learn to identify local frogs and toads. 3. Learn to use a taxonomic key. Today’s lab is the second in which you will learn about amphibian diversity. We will cover the Anura, or frogs and toads, the third and final clade of Lissamphibia. Tips for learning the material Continue what you have been doing in previous weeks. Examine all of the specimens on display, taking notes, drawings and photos of what you see. Attempt to identify the local species to species and the others to their higher clades. Quiz each other to see which taxa are easy for you and which ones give you troubles, and then revisit the difficult ones. Although the Anura has a conserved body plan – all are rather short and rigid bodied, with well- developed limbs, there is an incredible amount of diversity. Pay close attention to some of the special external anatomical traits that characterize the groups of frogs you see today. You will also learn to use a taxonomic key today. This is an important tool for correctly identifying species, especially when they are very difficult to distinguish from other species. 1 Ecol 483/583 – Lab 3: Anura 2010 Exercise 1: Anura diversity General Information Frogs are a monophyletic group comprising the order Anura. Salientia includes both extant and extinct frogs. Frogs have been around since the Triassic (~230 ma). -

Tetrapod Biostratigraphy and Biochronology of the Triassic–Jurassic Transition on the Southern Colorado Plateau, USA
Palaeogeography, Palaeoclimatology, Palaeoecology 244 (2007) 242–256 www.elsevier.com/locate/palaeo Tetrapod biostratigraphy and biochronology of the Triassic–Jurassic transition on the southern Colorado Plateau, USA Spencer G. Lucas a,⁎, Lawrence H. Tanner b a New Mexico Museum of Natural History, 1801 Mountain Rd. N.W., Albuquerque, NM 87104-1375, USA b Department of Biology, Le Moyne College, 1419 Salt Springs Road, Syracuse, NY 13214, USA Received 15 March 2006; accepted 20 June 2006 Abstract Nonmarine fluvial, eolian and lacustrine strata of the Chinle and Glen Canyon groups on the southern Colorado Plateau preserve tetrapod body fossils and footprints that are one of the world's most extensive tetrapod fossil records across the Triassic– Jurassic boundary. We organize these tetrapod fossils into five, time-successive biostratigraphic assemblages (in ascending order, Owl Rock, Rock Point, Dinosaur Canyon, Whitmore Point and Kayenta) that we assign to the (ascending order) Revueltian, Apachean, Wassonian and Dawan land-vertebrate faunachrons (LVF). In doing so, we redefine the Wassonian and the Dawan LVFs. The Apachean–Wassonian boundary approximates the Triassic–Jurassic boundary. This tetrapod biostratigraphy and biochronology of the Triassic–Jurassic transition on the southern Colorado Plateau confirms that crurotarsan extinction closely corresponds to the end of the Triassic, and that a dramatic increase in dinosaur diversity, abundance and body size preceded the end of the Triassic. © 2006 Elsevier B.V. All rights reserved. Keywords: Triassic–Jurassic boundary; Colorado Plateau; Chinle Group; Glen Canyon Group; Tetrapod 1. Introduction 190 Ma. On the southern Colorado Plateau, the Triassic– Jurassic transition was a time of significant changes in the The Four Corners (common boundary of Utah, composition of the terrestrial vertebrate (tetrapod) fauna. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -
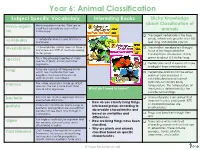
Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge About Classification of Micro-Organi Micro-Organisms Are Tiny
Year 6: Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge about Classification of micro-organi Micro-organisms are tiny. They are so small they can only be seen with a animals sm microscope. ❑ The largest vertebrate is the blue A vertebrate animal is one that has a whale, which can grow to over 100 vertebrates backbone. feet long and 400,000 pounds. An Invertebrate animal does not have a invertebrates ❑ The smallest vertebrate is thought backbone and 97% of creatures belong to be a tiny frog called the to this group. Paedophryne amauensis. It only This is the grouping together of similar grows to about 0.3 inches long. species species of plant, animal and other organisms. ❑ Vertebrates tend to be much more intelligent than invertebrates. Fungi are a group of living organisms fungi which are classified in their own ❑ Vertebrate animals can be either kingdom. This means they are not warm or cold-blooded. A animals, plants, or bacteria. cold-blooded animal cannot The whole organism is made up of just maintain a constant body monera one cell. The cell is more basic than temperature. The temperature of cells of other organisms. What do I need to know? their body is determined by the outside surroundings. Bacteria are tiny little organisms that are An invertebrate is an animal that bacteria ❑ everywhere around us. • How do you classify living things does not have a backbone. 97% Protists are not animals, plants, fungi, or of all animal species are protista into broad groups according to bacteria. Many protists are so small that invertebrates. -

Biogeographic Analysis Reveals Ancient Continental Vicariance and Recent Oceanic Dispersal in Amphibians ∗ R
Syst. Biol. 63(5):779–797, 2014 © The Author(s) 2014. Published by Oxford University Press, on behalf of the Society of Systematic Biologists. All rights reserved. For Permissions, please email: [email protected] DOI:10.1093/sysbio/syu042 Advance Access publication June 19, 2014 Biogeographic Analysis Reveals Ancient Continental Vicariance and Recent Oceanic Dispersal in Amphibians ∗ R. ALEXANDER PYRON Department of Biological Sciences, The George Washington University, 2023 G Street NW, Washington, DC 20052, USA; ∗ Correspondence to be sent to: Department of Biological Sciences, The George Washington University, 2023 G Street NW, Washington, DC 20052, USA; E-mail: [email protected]. Received 13 February 2014; reviews returned 17 April 2014; accepted 13 June 2014 Downloaded from Associate Editor: Adrian Paterson Abstract.—Amphibia comprises over 7000 extant species distributed in almost every ecosystem on every continent except Antarctica. Most species also show high specificity for particular habitats, biomes, or climatic niches, seemingly rendering long-distance dispersal unlikely. Indeed, many lineages still seem to show the signature of their Pangaean origin, approximately 300 Ma later. To date, no study has attempted a large-scale historical-biogeographic analysis of the group to understand the distribution of extant lineages. Here, I use an updated chronogram containing 3309 species (~45% of http://sysbio.oxfordjournals.org/ extant diversity) to reconstruct their movement between 12 global ecoregions. I find that Pangaean origin and subsequent Laurasian and Gondwanan fragmentation explain a large proportion of patterns in the distribution of extant species. However, dispersal during the Cenozoic, likely across land bridges or short distances across oceans, has also exerted a strong influence. -

Non-Native Small Terrestrial Vertebrates in the Galapagos 2 3 Diego F
1 Non-Native Small Terrestrial Vertebrates in the Galapagos 2 3 Diego F. Cisneros-Heredia 4 5 Universidad San Francisco de Quito USFQ, Colegio de Ciencias Biológicas y Ambientales, Laboratorio de Zoología 6 Terrestre & Museo de Zoología, Quito 170901, Ecuador 7 8 King’s College London, Department of Geography, London, UK 9 10 Email address: [email protected] 11 12 13 14 Introduction 15 Movement of propagules of a species from its current range to a new area—i.e., extra-range 16 dispersal—is a natural process that has been fundamental to the development of biogeographic 17 patterns throughout Earth’s history (Wilson et al. 2009). Individuals moving to new areas usually 18 confront a different set of biotic and abiotic variables, and most dispersed individuals do not 19 survive. However, if they are capable of surviving and adapting to the new conditions, they may 20 establish self-sufficient populations, colonise the new areas, and even spread into nearby 21 locations (Mack et al. 2000). In doing so, they will produce ecological transformations in the 22 new areas, which may lead to changes in other species’ populations and communities, speciation 23 and the formation of new ecosystems (Wilson et al. 2009). 24 25 Human extra-range dispersals since the Pleistocene have produced important distribution 26 changes across species of all taxonomic groups. Along our prehistory and history, we have aided 27 other species’ extra-range dispersals either by deliberate translocations or by ecological 28 facilitation due to habitat changes or modification of ecological relationships (Boivin et al. 29 2016). -

Phylogenetic Analyses Reveal Unexpected Patterns in the Evolution of Reproductive Modes in Frogs
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2012.01715.x PHYLOGENETIC ANALYSES REVEAL UNEXPECTED PATTERNS IN THE EVOLUTION OF REPRODUCTIVE MODES IN FROGS Ivan Gomez-Mestre,1,2 Robert Alexander Pyron,3 and John J. Wiens4 1Estacion´ Biologica´ de Donana,˜ Consejo Superior de Investigaciones Cientıficas,´ Avda. Americo Vespucio s/n, Sevilla 41092, Spain 2E-mail: [email protected] 3Department of Biological Sciences, The George Washington University, Washington DC 20052 4Department of Ecology and Evolution, Stony Brook University, Stony Brook, New York 11794–5245 Received November 2, 2011 Accepted June 2, 2012 Understanding phenotypic diversity requires not only identification of selective factors that favor origins of derived states, but also factors that favor retention of primitive states. Anurans (frogs and toads) exhibit a remarkable diversity of reproductive modes that is unique among terrestrial vertebrates. Here, we analyze the evolution of these modes, using comparative methods on a phylogeny and matched life-history database of 720 species, including most families and modes. As expected, modes with terrestrial eggs and aquatic larvae often precede direct development (terrestrial egg, no tadpole stage), but surprisingly, direct development evolves directly from aquatic breeding nearly as often. Modes with primitive exotrophic larvae (feeding outside the egg) frequently give rise to direct developers, whereas those with nonfeeding larvae (endotrophic) do not. Similarly, modes with eggs and larvae placed in locations protected from aquatic predators evolve frequently but rarely give rise to direct developers. Thus, frogs frequently bypass many seemingly intermediate stages in the evolution of direct development. We also find significant associations between terrestrial reproduction and reduced clutch size, larger egg size, reduced adult size, parental care, and occurrence in wetter and warmer regions. -

The Internet-Based Southeast Asia Amphibian Pet Trade
Rebecca E. Choquette et al. THE INTERNET-BASED SOUTHEAST ASIA AMPHIBIAN PET TRADE by Rebecca E. Choquette Ariadne Angulo Phillip J. Bishop Chi T. B. Phan Jodi J. L. Rowley © BROOBAS/CC BY-SA 4.0 © BROOBAS/CC BY-SA Polypedates otilophus Amphibians, as a class, are the most threatened vertebrates on the planet, with 41% of species threatened with extinction. Southeast Asian amphibian species in particular have been impacted by a high rate of habitat loss, and overharvesting for consumption, traditional medicine, and the pet trade has placed further pressure on populations. Collection for the pet trade is a online availability and demand for the pet trade of Southeast Asian amphibian species. We found postings for 59 Southeast Asian posts associated with the United Kingdom, the Czech Republic, the United States, Russia, and Germany. We highlight several species 68 TRAFFIC Bulletin Rebecca E. Choquette et al. The internet-based Southeast Asian amphibian pet trade Aet METHODS alet al et alet al et al study. et al et al et al researchers. Amphibian Species of the World et alet al et al et al et al et alet alet al. et al Yuan et al et al et alet al TRAFFIC Bulletin -

The Breeding Behavior of Glyphoglossus Molossus and the Tadpoles of Glyphoglossus Molossus and Calluella Guttulata (Microhylidae)
Zootaxa 3811 (3): 381–386 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3811.3.9 http://zoobank.org/urn:lsid:zoobank.org:pub:A449DA92-EA8B-4D8B-A538-C2845B33033F The breeding behavior of Glyphoglossus molossus and the tadpoles of Glyphoglossus molossus and Calluella guttulata (Microhylidae) RONALD ALTIG1, 3 & JODI J. L. ROWLEY2 1Department of Biological Sciences. Mississippi State University, Mississippi State, MS 39762, USA 2Australian Museum Research Institute, Australian Museum, 6 College Street, Sydney, NSW, 2010, Australia 3Corresponding author. E-mail: [email protected] Abstract The breeding behavior of Glyphoglossus molossus is described from still and video images taken in Cambodia. These large, burrowing frogs follow the general theme of microhylids that deposit aquatic eggs: explosive breeding in ephemeral water and performing multiple amplectic dips to oviposit surface films of pigmented eggs. A portion of a clutch is released with each dip, a dip lasts for about 6 s, 200-300 eggs are released per dip, and about 5 s pass between dips. The ova have a dark black animal pole and yellow vegetal pole. Expanded datasets on the morphology of the tadpoles of Glyphoglossus from Vietnam and Calluella from Myanmar are presented. Key words: Microhylidae, breeding, oviposition, tadpole Introduction There are rotund, short-legged frogs in several families (Nomura et al. 2009) that spend the majority of their time underground but emerge with seasonal rains to breed. Microhylid frogs that lay aquatic eggs commonly behave in this way, and the tadpoles are suspension feeders with depressed bodies, a reduced oral apparatus without keratinized mouthparts, lateral eyes, and a midventral spiracle.