Local and Landscape Effects on Arthropod Communities Along an Arable-Urban Gradient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Skipper Butterfly (Thymelicus Lineola) Associated
European Skipper Butterfly ( Thymelicus lineola ) Associated with Reduced Seed Development of Showy Lady’s-slipper Orchid (Cypripedium reginae ) PETER W. H ALL 1, 5, P AUL M. C ATLING 2, P AUL L. M OSQUIN 3, and TED MOSQUIN 4 124 Wendover Avenue, Ottawa, Ontario K1S 4Z7 Canada 2170 Sanford Avenue, Ottawa, Ontario K2C 0E9 Canada 3Research Triangle International, 3040 East Cornwallis Road, P.O. Box 12194, Raleigh, North Carolina 27709-2194 USA 43944 McDonalds Corners Road, Balderson, Ontario K0G 1A0 Canada 5Corresponding author: [email protected] Hall, Peter W., Paul M. Catling, Paul L. Mosquin, and Ted Mosquin. 2017. European Skipper butterfly ( Thymelicus lineola ) associated with reduced seed development of Showy Lady’s-slipper orchid ( Cypripedium reginae ). Canadian Field- Naturalist 131(1): 63 –68. https://doi.org/10.22621/cfn.v 131i 1. 1952 It has been suggested that European Skipper butterflies ( Thymelicus lineola ) trapped in the lips of the Showy Lady’s-slipper orchid ( Cypripedium reginae ) may interfere with pollination. This could occur through blockage of the pollinator pathway, facilitation of pollinator escape without pollination, and/or disturbance of the normal pollinators. A large population of the orchid at an Ottawa Valley site provided an opportunity to test the interference hypothesis. The number of trapped skippers was compared in 475 post-blooming flowers with regard to capsule development and thus seed development. The presence of any skippers within flowers was associated with reduced capsule development ( P = 0.0075), and the probability of capsule development was found to decrease with increasing numbers of skippers ( P = 0.0271). The extent of a negative effect will depend on the abundance of the butterflies and the coincidence of flowering time and other factors. -

Bus Linie 186 Fahrpläne & Karten
Bus Linie 186 Fahrpläne & Netzkarten 186 Bovenden Schulzentrum - Adelebsen Schule Im Website-Modus Anzeigen Die Bus Linie 186 (Bovenden Schulzentrum - Adelebsen Schule) hat 5 Routen (1) Adelebsen: 13:09 - 15:35 (2) Bovenden Schulzentrum: 07:10 - 08:10 (3) Emmenhausen: 12:25 (4) Harste: 07:49 - 13:04 (5) Lenglern: 07:37 - 13:11 Verwende Moovit, um die nächste Station der Bus Linie 186 zu ƒnden und, um zu erfahren wann die nächste Bus Linie 186 kommt. Richtung: Adelebsen Bus Linie 186 Fahrpläne 17 Haltestellen Abfahrzeiten in Richtung Adelebsen LINIENPLAN ANZEIGEN Montag Kein Betrieb Dienstag Kein Betrieb Bovenden Schulzentrum Osterberg 2a, Bovenden Mittwoch Kein Betrieb Bovenden Rathaus Donnerstag 13:09 - 15:35 Bovenden Rathaus, Bovenden Freitag Kein Betrieb Bovenden Feldtorweg Samstag Kein Betrieb Bovenden Feldtorweg, Bovenden Sonntag Kein Betrieb Bovenden Breite Straße Göttinger Straße, Bovenden Lenglern Schule Brandenburger Straße 14, Germany Bus Linie 186 Info Richtung: Adelebsen Lenglern Bahnhof Stationen: 17 L 556, Germany Fahrtdauer: 32 Min Linien Informationen: Bovenden Schulzentrum, Harste Neustadt Bovenden Rathaus, Bovenden Feldtorweg, Bovenden Neustadt 3, Germany Breite Straße, Lenglern Schule, Lenglern Bahnhof, Harste Neustadt, Harste Westbergsweg, Harste Westbergsweg Emmenhausen An Der Harste, Emmenhausen Hauptstraße 35, Germany Harster Straße, Erbsen Fehrlingser Weg, Erbsen In Der Straut, Lödingsen Gartenstraße, Lödingsen Emmenhausen An Der Harste Lindenallee, Adelebsen Bergstraße, Adelebsen Berghofstraße, Germany Angerstraße, -

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

THE HUMBLE-BEE MACMILLAN and CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE the MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO the MACMILLAN CO
THE HUMBLE-BEE MACMILLAN AND CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE THE MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO THE MACMILLAN CO. OF CANADA, Ltd. TORONTO A PET QUEEN OF BOMBUS TERRESTRIS INCUBATING HER BROOD. (See page 139.) THE HUMBLE-BEE ITS LIFE-HISTORY AND HOW TO DOMESTICATE IT WITH DESCRIPTIONS OF ALL THE BRITISH SPECIES OF BOMBUS AND PSITHTRUS BY \ ; Ff W. U SLADEN FELLOW OF THE ENTOMOLOGICAL SOCIETY OF LONDON AUTHOR OF 'QUEEN-REARING IN ENGLAND ' ILLUSTRATED WITH PHOTOGRAPHS AND DRAWINGS BY THE AUTHOR AND FIVE COLOURED PLATES PHOTOGRAPHED DIRECT FROM NA TURE MACMILLAN AND CO., LIMITED ST. MARTIN'S STREET, LONDON 1912 COPYRIGHT Printed in ENGLAND. PREFACE The title, scheme, and some of the contents of this book are borrowed from a little treatise printed on a stencil copying apparatus in August 1892. The boyish effort brought me several naturalist friends who encouraged me to pursue further the study of these intelligent and useful insects. ..Of these friends, I feel especially indebted to the late Edward Saunders, F.R.S., author of The Hymen- optera Aculeata of the British Islands, and to the late Mrs. Brightwen, the gentle writer of Wild Nattcre Won by Kindness, and other charming studies of pet animals. The general outline of the life-history of the humble-bee is, of course, well known, but few observers have taken the trouble to investigate the details. Even Hoffer's extensive monograph, Die Htimmeln Steiermarks, published in 1882 and 1883, makes no mention of many remarkable can particulars that I have witnessed, and there be no doubt that further investigations will reveal more. -

Bombus Terrestris L
Apidologie 39 (2008) 419–427 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2008020 Original article Foraging distance in Bombus terrestris L. (Hymenoptera: Apidae)* Stephan Wolf, Robin F.A. Moritz Institut für Biologie / Institutsbereich Zoologie, Martin-Luther-Universität Halle-Wittenberg, Germany Received 11 October 2007 – Revised 7 February 2008 – Accepted 25 February 2008 Abstract – A major determinant of bumblebees pollination efficiency is the distance of pollen dispersal, which depends on the foraging distance of workers. We employ a transect setting, controlling for both forage and nest location, to assess the foraging distance of Bombus terrestris workers and the influence of environmental factors on foraging frequency over distance. The mean foraging distance of B. terrestris workers was 267.2 m ± 180.3 m (max. 800 m). Nearly 40% of the workers foraged within 100 m around the nest. B. terrestris workers have thus rather moderate foraging ranges if rewarding forage is available within vicinity of the nests. We found the spatial distribution and the quality of forage plots to be the major determinants for the bees foraging decision-making, explaining over 80% of the foraging frequency. This low foraging range has implications for using B. terrestris colonies as pollinators in agriculture. Bumblebee / foraging / pollination / decision-making 1. INTRODUCTION efficiency (Gauld et al., 1990; Westerkamp, 1991; Wilson and Thomson, 1991; Goulson, Pollen dispersal through animal pollinators 2003). This is partly due to the more robust is essential for plant reproduction. The effi- handling of flowers by bumblebees and their ciency of pollinators depends on various fac- ability of buzz-pollination (e.g. -
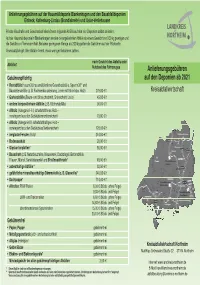
Deponiegebühren
Anlieferungsgebühren auf der Hausmülldeponie Blankenhagen und den Bauabfalldeponien Einbeck, Katlenburg-Lindau (Brandisbreite) und Uslar-Verliehausen Private Haushalte und Gewerbebetriebe können folgende Abfälle auf den vier Deponien selbst anliefern. Auf der Hausmülldeponie in Blankenhagen werden die angelieferten Abfälle ab einem Gewicht von 200 kg gewogen und die Gebühr pro Tonne ermittelt. Bei einer geringeren Menge als 200 kg gelten die Gebühren auf der Rückseite. Grundsätzlich gilt: Wer Abfälle trennt, muss weniger Gebühren zahlen. nach Gewicht des Abfalls oder Abfallart Nutzlast des Fahrzeuges Anlieferungsgebühren Gebührenpflichtig auf den Deponien ab 2021 • Restabfälle Hausmüll, hausmüllähnliche Gewerbeabfälle, Sperrmüll** und Baustellenabfälle (z. B Fachwerkauskleidung, Lehm mit Stroh bzw. Holz) 279,00 €/t Kreisabfallwirtschaft • Gartenabfälle (Baum- und Strauchschnitt, Grünschnitt, Laub) 45,00 €/t • andere kompostierbare Abfälle (z. B. Küchenabfälle) 90,00 €/t • Altholz (Kategorie I–III, schadstofffreies Holz – vorwiegend aus den Gebäudeinnenbereichen) 79,00 €/t • Altholz (Kategorie IV, schadstoffhaltiges Holz – vorwiegend aus den Gebäudeaußenbereichen) 128,00 €/t • verglaste Fenster (Holz) 244,00 €/t • Bodenaushub 20,00 €/t • Gipskartonplatten* 66,00 €/t • Bauschutt (z. B. Naturbausteine, Mauerwerk, Dachziegel, Betonabfälle, Fliesen, Mörtel, Sanitärkeramik) und Straßenaufbruch* 48,00 €/t • asbesthaltige Abfälle * 92,50 €/t • gefährliche mineralfaserhaltige Dämmstoffe (z. B. Glaswolle)* 240,00 €/t • Dachpappe* 714,00 €/t • Altreifen: -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -

Eastern Persius Duskywing Erynnis Persius Persius
COSEWIC Assessment and Status Report on the Eastern Persius Duskywing Erynnis persius persius in Canada ENDANGERED 2006 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and status report on the Eastern Persius Duskywing Erynnis persius persius in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 41 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge M.L. Holder for writing the status report on the Eastern Persius Duskywing Erynnis persius persius in Canada. COSEWIC also gratefully acknowledges the financial support of Environment Canada. The COSEWIC report review was overseen and edited by Theresa B. Fowler, Co-chair, COSEWIC Arthropods Species Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’Hespérie Persius de l’Est (Erynnis persius persius) au Canada. Cover illustration: Eastern Persius Duskywing — Original drawing by Andrea Kingsley ©Her Majesty the Queen in Right of Canada 2006 Catalogue No. CW69-14/475-2006E-PDF ISBN 0-662-43258-4 Recycled paper COSEWIC Assessment Summary Assessment Summary – April 2006 Common name Eastern Persius Duskywing Scientific name Erynnis persius persius Status Endangered Reason for designation This lupine-feeding butterfly has been confirmed from only two sites in Canada. -

Financial Statements Sartorius AG 2020
Sartorius AG Sartorius AG 2020 Financial Statements Contents 2 Contents Financial Statements and Notes 3 Balance Sheet as of December 31, 2020 4 Statement of Profit and Loss for the Period of January 1 to December 31, 2020 6 Notes to the Financial Statements for Fiscal 2020 7 Notes to the Individual Balance Sheet Items 10 Notes to the Statement of Profit and Loss 15 Other Disclosures 18 Declaration of the Executive Board 21 Independent Auditor‘s Report 22 Supplementary Information 30 Development of Fixed Assets 31 Share Ownership 33 Executive Board and Supervisory Board 36 About This Publication 44 Forward-looking Statements Contain Risks This annual report contains statements concerning the future performance of Sartorius AG. These statements are based on assumptions and estimates. Although we are convinced that these forward-looking statements are realistic, we cannot guarantee that they will actually apply. This is because our assumptions harbor risks and uncertainties that could lead to actual results diverging substantially from the expected ones. It is not planned to update our forward-looking statements. This is a translation of the original German-language financial statements. Sartorius shall not assume any liability for the correctness of this translation. The original German financial statements are the legally binding version. Furthermore, Sartorius reserves the right not to be responsible for the topicality, correctness, completeness or quality of the information provided. Liability claims regarding damage caused by the use of any information provided, including any kind of information which is incomplete or incorrect, will therefore be rejected. Throughout these financial statements, differences may be apparent as a result of rounding during addition. -

Amtsblatt Für Den Landkreis Northeim
Amtsblatt für den Landkreis Northeim Jahrgang 2020 Northeim, den 16.12.2020 Nr. 74 Inhalt: A. Amtliche Bekanntmachungen des Landkreises Vereinbarung über die Erhebung von Entgelten im Rettungsdienst gemäß § 15 des Niedersächsischen Rettungsdienstgesetzes Verordnung über das Landschaftsschutzgebiet „Schwülme“ vom 04.12.2020 Übersichtskarte „Schwülme“ III. Nachtrag zur Satzung vom 10.03.2017 über die Erhebung von Gebühren für die Abfallbewirtschaftung B. Amtliche Bekanntmachungen der Städte und Gemeinden Stadt Moringen Berichtigung des Flächennutzungsplanes im Zuge der Aufstellung des Bebauungsplanes Nr. 29 „An der Specke“ Gebührensatzung zur Satzung über das Friedhofs- und Bestattungswesen Flecken Bodenfelde Hundesteuersatzung Gemeinde Kalefeld 4. Nachtrag über die Erhebung von Verwaltungskosten im eigenen Wirkungskreis (Verwaltungskostensatzung) _____________________________________________________________________ Herausgeber: Landkreis Northeim, Medenheimer Str. 6 –8, 37154 Northeim Erscheint grundsätzlich jeden Mittwoch (außer feiertags), Redaktionsschluss ist jeweils dienstags 16.00 Uhr Auskunft, Einsichtnahme und Einzelexemplare: Frau Keufner, Personalratsassistenz, Tel. 05551-708-238, oder Frau Topel-Bohnhorst, Tel. 05551/708-0, E-Mail: [email protected] Das Amtsblatt kann auf der Internetseite www.landkreis-northeim.de kostenlos eingesehen werden. - 2 - Gemeinde Katlenburg-Lindau Öffentliche Bekanntmachung des Jahresabschlusses 2019 und des konsolidierten Gesamtabschlusses 2018 XIV. Nachtrag zur Satzung über die Erhebung -

Hoverfly Newsletter 67
Dipterists Forum Hoverfly Newsletter Number 67 Spring 2020 ISSN 1358-5029 . On 21 January 2020 I shall be attending a lecture at the University of Gloucester by Adam Hart entitled “The Insect Apocalypse” the subject of which will of course be one that matters to all of us. Spreading awareness of the jeopardy that insects are now facing can only be a good thing, as is the excellent number of articles that, despite this situation, readers have submitted for inclusion in this newsletter. The editorial of Hoverfly Newsletter No. 66 covered two subjects that are followed up in the current issue. One of these was the diminishing UK participation in the international Syrphidae symposia in recent years, but I am pleased to say that Jon Heal, who attended the most recent one, has addressed this matter below. Also the publication of two new illustrated hoverfly guides, from the Netherlands and Canada, were announced. Both are reviewed by Roger Morris in this newsletter. The Dutch book has already proved its value in my local area, by providing the confirmation that we now have Xanthogramma stackelbergi in Gloucestershire (taken at Pope’s Hill in June by John Phillips). Copy for Hoverfly Newsletter No. 68 (which is expected to be issued with the Autumn 2020 Dipterists Forum Bulletin) should be sent to me: David Iliff, Green Willows, Station Road, Woodmancote, Cheltenham, Glos, GL52 9HN, (telephone 01242 674398), email:[email protected], to reach me by 20 June 2020. The hoverfly illustrated at the top right of this page is a male Leucozona laternaria. -

Bumblebee in the UK
There are 24 species of bumblebee in the UK. This field guide contains illustrations and descriptions of the eight most common species. All illustrations 1.5x actual size. There has been a marked decline in the diversity and abundance of wild bees across Europe in recent decades. In the UK, two species of bumblebee have become extinct within the last 80 years, and seven species are listed in the Government’s Biodiversity Action Plan as priorities for conservation. This decline has been largely attributed to habitat destruction and fragmentation, as a result of Queen Worker Male urbanisation and the intensification of agricultural practices. Common The Centre for Agroecology and Food Security is conducting Tree bumblebee (Bombus hypnorum) research to encourage and support bumblebees in food Bumblebees growing areas on allotments and in gardens. Bees are of the United Kingdom Queens, workers and males all have a brown-ginger essential for food security, and are regarded as the most thorax, and a black abdomen with a white tail. This important insect pollinators worldwide. Of the 100 crop species that provide 90% of the world’s food, over 70 are recent arrival from France is now present across most pollinated by bees. of England and Wales, and is thought to be moving northwards. Size: queen 18mm, worker 14mm, male 16mm The Centre for Agroecology and Food Security (CAFS) is a joint initiative between Coventry University and Garden Organic, which brings together social and natural scientists whose collective research expertise in the fields of agriculture and food spans several decades. The Centre conducts critical, rigorous and relevant research which contributes to the development of agricultural and food production practices which are economically sound, socially just and promote long-term protection of natural Queen Worker Male resources.