Paleobotanical Studies of the Appian Way Fossil Locality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peter H. Griggs
STRATIGRAPHIC SIGNIFICANCE OF FOSSIL POLLEN AND SPORES OF THE CHUCKANUT FORMATION, NORTHWEST WASHINGTON Thosis far the Degree of. M. S. MICHIGAN STATE UNIVERSI'I’Y Peter H. Griggs 1965 ~-.. 'Wfi— wwWWL ‘1. .Illilllflu‘fllllf -Jnl.| IEI‘II‘I'IIIIVFI (II 'llllll‘lll.‘ I, l {III-III! I’lII 'II (III! III, [I , . III! [III [I I'll! (III. (I ABSTRACT STRATIGRAPHIC SIGNIFICANCE OF FOSSIL POLLEN AND SPORES OF THE CHUCKANUT FORMATION, NORTHWEST WASHINGTON by Peter H. Griggs The Chuckanut Formation, a series of strongly folded, terrestrial sandstones and shales, outcrops in northwestern Washington. The rocks have been previously assigned an age of Upper Cretaceous to lower Eocene based upon plant megafossils. The microfossil flora of the standard section along the east shore of Samish Bay was examined in order to determine the relationship of the Chuckanut to the Burrard (middle Eocene) of British Columbia. A zonation and envir- onmental interpretation of the standard section was desired for further refinement of the geological history of Tertiary rocks in the Pacific Northwest. Twenty—two samples, representing 9,H8u feet of section, were examined for palynomorphs. Data were collected for both relative frequency and stratigraphic analysis. Seventy— nine palynomorphs are described. The Samish Bay section is divided into three zones. The zonation is based on changes in the relative frequency f and the stratigraphic range of the palynomorphs. These ’ '_ ‘ V'II -7 r1 ' < P812631 ALL. '.,I I» ) I— (1 0'). OT changes were brought about by changing environmental and climatic conditions during the deposition of the rocks. An age of Paleocene to lower Eocene is assigned to the Samish Bay section. -

VOLCANIC INFLUENCE OVER FLUVIAL SEDIMENTATION in the CRETACEOUS Mcdermott MEMBER, ANIMAS FORMATION, SOUTHWESTERN COLORADO
VOLCANIC INFLUENCE OVER FLUVIAL SEDIMENTATION IN THE CRETACEOUS McDERMOTT MEMBER, ANIMAS FORMATION, SOUTHWESTERN COLORADO Colleen O’Shea A Thesis Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August: 2009 Committee: James Evans, advisor Kurt Panter, co-advisor John Farver ii Abstract James Evans, advisor Volcanic processes during and after an eruption can impact adjacent fluvial systems by high influx rates of volcaniclastic sediment, drainage disruption, formation and failure of natural dams, changes in channel geometry and changes in channel pattern. Depending on the magnitude and frequency of disruptive events, the fluvial system might “recover” over a period of years or might change to some other morphology. The goal of this study is to evaluate the preservation potential of volcanic features in the fluvial environment and assess fluvial system recovery in a probable ancient analog of a fluvial-volcanic system. The McDermott Member is the lower member of the Late Cretaceous - Tertiary Animas Formation in SW Colorado. Field studies were based on a southwest-northeast transect of six measured sections near Durango, Colorado. In the field, 13 lithofacies have been identified including various types of sandstones, conglomerates, and mudrocks interbedded with lahars, mildly reworked tuff, and primary pyroclastic units. Subsequent microfacies analysis suggests the lahar lithofacies can be subdivided into three types based on clast composition and matrix color, this might indicate different volcanic sources or sequential changes in the volcanic center. In addition, microfacies analysis of the primary pyroclastic units suggests both surge and block-and-ash types are present. -

Flowering Plants; They Were Too Numerous and Too Varied, and There Were Too Few Fos- Sils to Sort out Which Were More Primitive
NEWSFOCUS embryo that serves as its food supply. Darwin was perplexed by the diversity of On the Origin of flowering plants; they were too numerous and too varied, and there were too few fos- sils to sort out which were more primitive. Flowering Plants Throughout much of the 20th century, mag- nolia relatives with relatively large flowers were leading candidates for the most primi- how flowers got started—and from which tive living flowers, although a few ancestor. Today, researchers have analytical researchers looked to small herbs instead. tools, fossils, genomic data, and insights that In the late 1990s, molecular systematics Darwin could never have imagined, all of came to the rescue, with several reports pre- which make these mysteries less abom- senting a fairly consistent picture of the inable. Over the past 40 years, techniques lower branches of the angiosperm tree. An for assessing the relationships between obscure shrub found only in New Caledonia organisms have greatly improved, and gene emerged as a crucial window to the past. sequences, as well as morphology, now help Amborella trichopoda, with its 6-millimeter researchers sort out which angiosperms greenish-yellow flowers, lives deep in the arose early and which arose late. New fossil cloud forests there. In multiple gene-based finds and new ways to study them—with assessments, including an analysis in 2007 synchrotron radiation, for example—pro- of 81 genes from chloroplast genomes vide a clearer view of the detailed anatomy belonging to 64 species, Amborella sits of ancient plants. And researchers from var- at the base of the angiosperm family tree, ious fields are figuring out genomic changes the sister group of all the rest of the that might explain the amazing success of angiosperms. -

South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae) Lendel, Anita Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-93287 Dissertation Published Version Originally published at: Lendel, Anita. South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). 2013, University of Zurich, Faculty of Science. South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae) _________________________________________________________________________________ Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr.sc.nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Anita Lendel aus Kroatien Promotionskomitee: Prof. Dr. H. Peter Linder (Vorsitz) PD. Dr. Reto Nyffeler Prof. Dr. Elena Conti Zürich, 2013 Table of Contents Acknowledgments 1 Introduction 3 Chapter 1. Phylogenetics and taxonomy of the tribe Cereeae s.l., with particular focus 15 on the subtribe Trichocereinae (Cactaceae – Cactoideae) Chapter 2. Floral evolution in the South American tribe Cereeae s.l. (Cactaceae: 53 Cactoideae): Pollination syndromes in a comparative phylogenetic context Chapter 3. Contemporaneous and recent radiations of the world’s major succulent 86 plant lineages Chapter 4. Tackling the molecular dating paradox: underestimated pitfalls and best 121 strategies when fossils are scarce Outlook and Future Research 207 Curriculum Vitae 209 Summary 211 Zusammenfassung 213 Acknowledgments I really believe that no one can go through the process of doing a PhD and come out without being changed at a very profound level. -

Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002
Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Kadoorie Farm and Botanic Garden in collaboration with Guangxi Zhuang Autonomous Region Forestry Department Guangxi Forestry Survey and Planning Institute South China Institute of Botany South China Normal University Institute of Zoology, CAS March 2003 South China Forest Biodiversity Survey Report Series: No. 27 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Cenwanglaoshan Nature Reserve, Northwest Guangxi, China, 1999 and 2002 Editors John R. Fellowes, Bosco P.L. Chan, Michael W.N. Lau, Ng Sai-Chit and Gloria L.P. Siu Contributors Kadoorie Farm and Botanic Garden: Gloria L.P. Siu (GS) Bosco P.L. Chan (BC) John R. Fellowes (JRF) Michael W.N. Lau (ML) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Graham T. Reels (GTR) Roger C. Kendrick (RCK) Guangxi Zhuang Autonomous Region Forestry Department: Xu Zhihong (XZH) Pun Fulin (PFL) Xiao Ma (XM) Zhu Jindao (ZJD) Guangxi Forestry Survey and Planning Institute (Comprehensive Tan Wei Fu (TWF) Planning Branch): Huang Ziping (HZP) Guangxi Natural History Museum: Mo Yunming (MYM) Zhou Tianfu (ZTF) South China Institute of Botany: Chen Binghui (CBH) Huang Xiangxu (HXX) Wang Ruijiang (WRJ) South China Normal University: Li Zhenchang (LZC) Chen Xianglin (CXL) Institute of Zoology CAS (Beijing): Zhang Guoqing (ZGQ) Chen Deniu (CDN) Nanjing University: Chen Jianshou (CJS) Wang Songjie (WSJ) Xinyang Teachers’ College: Li Hongjing (LHJ) Voluntary specialist: Keith D.P. Wilson (KW) Background The present report details the findings of visits to Northwest Guangxi by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme. -

Paisia, an Early Cretaceous Eudicot Angiosperm Flower With
Grana, 2018 Vol. 57, Nos. 1–2, 1–15, https://doi.org/10.1080/00173134.2017.1310292 Paisia, an Early Cretaceous eudicot angiosperm flower with pantoporate pollen from Portugal ELSE MARIE FRIIS 1, MÁRIO MIGUEL MENDES 2,3 & KAJ RAUNSGAARD PEDERSEN 4 1Department of Palaeobiology, Swedish Museum of Natural History, Stockholm, Sweden, 2Centre for Interdisciplinary Development and Research on Environment, Applied Management and Space, Lusófona University of Humanities and Technologies, Lisboa, Portugal, 3Centre for Marine and Environmental Research, University of Algarve, Campus de Gambelas, Faro, Portugal, 4Department of Geosciences, University of Aarhus, Aarhus, Denmark Abstract A new fossil angiosperm, Paisia pantoporata, is described from the Early Cretaceous Catefica mesofossil flora, Portugal, based on coalified floral buds, flowers and isolated floral structures. The flowers are actinomorphic and structurally bisexual with a single whorl of five fleshy tepals, a single whorl of five stamens and a single whorl of five carpels. Tepals, stamens and carpels are opposite, arranged on the same radii and tepals are involute at the base clasping the stamens. Stamens have a massive filament that grades without a joint into the anther. The anthers are dithecate and tetraspor- angiate with extensive connective tissue between the tiny pollen sacs. Pollen grains are pantoporate and spiny. The carpels are free, apparently plicate, with many ovules borne in two rows along the ventral margins. Paisia pantoporata is the oldest known flower with pantoporate pollen. Similar pantoporate pollen was also recognised in the associated dispersed palynoflora. Paisia is interpreted as a possibly insect pollinated, herbaceous plant with low pollen production and low dispersal potential of the pollen. -

BULLETINS of AMERICAN PALEONTOLOGY and PALAEONTOGRAPHICA AMERICANA
8'oL HARVARD UNIVERSITY |v 1 ^J^y^ MUS. COMP. BULLETINSZOoL ^ LIBRARY OF MAR 4 *97^ HAi UNJVERi^-AMERICAN PALEONTOLOGY VOL. LXIV 1973-74 Paleontological Research Institution Ithaca, New York 14850 U. S. A. In Memoriam Orville L. Bandy 1916-1973 CONTENTS OF VOLUME LXIV Bulletin No. Pages Plates 278. Palynology of the ASmond Formation (Upper Cretaceous) Rock Springs Uplift, Wyoming. By J. Fred Stone 1-136 1-20 279. Tabulate Corals and Echinoderms from the Pennsylvanian Winterset Limestone, Hog- shooter Formation, Northeastern Oklahoma. By H. L. Strimple and J. M. Cooke 137-168 21 280. Stratigraphy and Genera of Calcareous Foraminifera cf the Fraileys Fades (Missis- sippian) of Central Kentucky. By R. G. Browne and E. R. Pohl 169-244 22-31 281. Crinoid Studies. Part I. Some Pennsylvanian Crinoids from Nebraska. Part. II. Some Permian Crinoids from Nebraska, Kansas, and Oklahoma. By R. K. Pabian and H. L. Strimple 245-338 32-41 INDEX volume. Each number is No separate index is included in the listed in the begin- indexed separately. Contents of the volume are ning of the volume. BULLETINS MUS. COMP. 200U LIBRARY OF OCT 16 19^ AMERICAN t^S«?fALEONTOLOGY (Founded 1895) Vol. 64 No. 278 PALYNOLOGY OF THE ALMOND FORMATION (UPPER CRETACEOUS), ROCK SPRINGS UPLIFT, WYOMING By J. Fred Stone 1973 Paleontological Research Institution Ithaca, New York 14850, U.S.A. PALEONTOLOGIGAL RESEARCH INSTITUTION 1972 - 73 President Daniel B. Sass Vice-President Merrill W. Haas Secretary Philip C. Wakeley Assistant Secretary Rebecca S. Harris Director, Treasurer Katherine V. W. Palmer Counsel Armand L. Adams Representative AAAS Council John Pojeta, Jr. -

Isopogon & Petrophile
A U S T R AL I A N N A T I V E P L A N T S A S S O C I A T I O N ( A U S T ) Isopogon & Petrophile Study Group Newsletter No. 22 April 2018 ISSN 1445-9493 Website http://anpsa.org.au/iso-petSG/ STUDY GROUP LEADERS/NEWSLETTER EDITORS Catriona Bate & Phil Trickett Email: [email protected] Ph: 0409 789 567 Isopogon teretifolius, Hi Vallee farm, Badgingarra WA, October 2017. See page 10 for our profile of this species. Back issues of the Isopogon and Petrophile Study Group Newsletter are available at http://anpsa.org.au/iso-petSG/IPSG-news.html Isopogon & Petrophile Study Group Newsletter April 2018 1 In this issue Editorial From our members Exchanging cuttings and seed Galls galore Cranbourne I&P Special Collection Painting I. formosus Plant profile – I. teretifolius Plant profile – P. sessilis Dryandra Woodland WA: discovering P. circinata and I. villosus Learnings from Cranbourne Special Collection A seed germination diary: I. anethifolius and P. pulchella Grafting update Seed vs cuttings: P. pedunculata Growing WA natives: experiences in the east and the west Petrophile fossils? Two names discovered In the press Financial report Dear Members, The traditional hot, dry summer reputation of Australia seems to have struck with a vengeance this year. Members have reported little useful rainfall for the entire summer with inland NSW and Victoria/South Australia suffering through one of their driest summers on record. Even here on the normally wet South Coast of NSW, we have had our driest summer since we moved here in 2010. -

Geobotany Studies
Geobotany Studies Basics, Methods and Case Studies Editor Franco Pedrotti University of Camerino Via Pontoni 5 62032 Camerino Italy Editorial Board: S. Bartha, Va´cra´tot, Hungary F. Bioret, University of Brest, France E. O. Box, University of Georgia, Athens, Georgia, USA A. Cˇ arni, Slovenian Academy of Sciences, Ljubljana (Slovenia) K. Fujiwara, Yokohama City University, Japan D. Gafta, “Babes-Bolyai” University Cluj-Napoca (Romania) J. Loidi, University of Bilbao, Spain L. Mucina, University of Perth, Australia S. Pignatti, Universita degli Studi di Roma “La Sapienza”, Italy R. Pott, University of Hannover, Germany A. Vela´squez, Centro de Investigacion en Scie´ncias Ambientales, Morelia, Mexico R. Venanzoni, University of Perugia, Italy For further volumes: http://www.springer.com/series/10526 About the Series The series includes outstanding monographs and collections of papers on a given topic in the following fields: Phytogeography, Phytosociology, Plant Community Ecology, Biocoenology, Vegetation Science, Eco-informatics, Landscape Ecology, Vegetation Mapping, Plant Conservation Biology and Plant Diversity. Contributions are expected to reflect the latest theoretical and methodological developments or to present new applications at broad spatial or temporal scales that could reinforce our understanding of ecological processes acting at the phytocoenosis and landscape level. Case studies based on large data sets are also considered, provided that they support refinement of habitat classification, conservation of plant diversity, or -

The Yellowstone Paleontological Survey
E PALEONT ON O T LO S G W I O C L A L L E National Y Park The Yellowstone Service Department of the Interior Paleontological Survey SURVEY Vincent L. Santucci Yellowstone Center for Resources National Park Service Yellowstone National Park, Wyoming YCR-NR-98-1 1998 How to cite this document: Santucci, V. L. 1998. The Yellowstone Paleontological Survey. Yellowstone Center for Resources, National Park Service, Yellowstone National Park, Wyoming,YCR-NR-98-1. Current address for Vincent L. Santucci is National Park Service, P.O. Box 592, Kemmerer, WY 83101. The Yellowstone Paleontological Survey To Lt. Col. Luke J. Barnett, III “Uncle by blood, brother in spirit!” Vincent L. Santucci Yellowstone Center for Resources National Park Service Yellowstone National Park, Wyoming YCR-NR-98-1 1998 Table of Contents Introduction .................................................................................................... 1 Stratigraphy .................................................................................................... 4 Fossil Chronology........................................................................................... 6 Taxonomy ..................................................................................................... 12 Localities ...................................................................................................... 15 Interpretation ................................................................................................ 19 Paleontological Resource Management....................................................... -
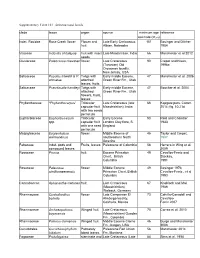
Supplementary Table 10.1. Selected Rosid Fossils Clade Taxon Organ Source Minimum Age Reference Estimate (Mya) Indet
Supplementary Table 10.1. Selected rosid fossils clade taxon organ source minimum age reference estimate (Mya) Indet. Rosidae Rose Creek flower Flower and Late Early Cretaceous 101 Basinger and Dilcher fruit Albian, Nebraska 1984 Vitaceae Indovitis chitaleyae fruit with intact Late Maastrictian, India 66 Manchester et al 2012 seeds Clusiaceae Paleoclusia chevalieri flower Late Cretaceous 90 Crepet and Nixon, (Turonian) Old 1998a Crossman locality, New Jersey, USA Salicaceae Populus tidwellii & P. Twigs with Early middle Eocene, 47 Manchester et al. 2006 wilmatae attached Green River Fm., Utah leaves, fruits Salicaceae Pseudosalix handleyi Twigs with Early middle Eocene, 47 Boucher et al. 2004 attached Green River Fm., Utah flowers, fruits, leaves Phyllanthaceae “Phyllanthocarpus” Trilocular Late Cretaceous (late 66 Kapgate pers. Comm. capsular fruit Maastrichtian), India 2014; fig. 10.21d with two seeds per locule Euphorbiaceae Euphorbiocarpon Trilocular Early Eocene 50 Reid and Chandler spp. capsular fruit London Clay flora, S. 1933 with one seed England per locule Malpighiaceae Eoglandulosa flower Middle Eocene of 45 Taylor and Crepet, warmanensis southeastern North 1987 America Fabaceae Indet. pods and Fruits, leaves Paleocene of Colombia 58 Herrera in Wing et al. compound leaves 2009 Rosaceae Prunus fruit Eocene Princeton 49 Cevallos-Ferriz and Chert, British Stockey, Columbia 1991 Rosaceae Paleorosa flower Middle Eocene 49 Basinger 1976; similkameenensis Princeton Chert, British Cevallos-Ferriz , et al Columbia 1993 Cannabaceae Aphananthe cretacea fruit Late Cretaceous 67 Knobloch and Mai (Maastrichtian) 1986 Walbeck, Germany Rhamnaceae Coahuilanthus flower Late Campanian El 73 Calvillo-Canadell and belinda Almácigo locality, Cevallos- Coahuila, Mexico Ferriz 2007 Rhamnaceae Archaeopaliurus Winged fruit Late Cretaceous 70 Correa et al. -

Acknowledgment of Reviewers, 2015
Acknowledgment of Reviewers, 2015 The PNAS editors would like to thank all the individuals who dedicated their considerable time and expertise to the journal by serving as reviewers in 2015. Their generous contribution is deeply appreciated. A Peter B. Adler Colin J. Akerman Eric E. Allen James Ammerman Duur K. Aanen Ralph Adolphs Joshua M. Akey Heather C. Allen David M. Amodio Adam R. Abate Ruedi Aebersold Anna Akhmanova Jim Allen Valentin Amrhein John T. Abatzoglou Hugo Aerts Hajime Akimoto Karen N. Allen Esther Amstad Jonathan Abbatt Hagit P. Affek Akin Akinc Michael F. Allen Ronald Amundson Allison Abbott Arash Afraz Shizuo Akira Paul M. Allen Weihua An Jeffrey Abbott Theodor Agapie Ozan Akkus Rosalind J. Allen Zhiqiang An Larry F. Abbott David A. Agard Ivona Aksentijevich Morten Erik Allentoft Laura Diaz Anadon Nicholas L. Abbott Sapan Agarwal Serap Aksoy Stefano Allesina Ganesh Srinivasan Anand Chaouki T. Abdallah Joel W. Ager III Yousef Al-Abed David B. Allison Cort Anastasio Omar Abdel-Wahab Ingi Agnarsson Ashraf Al-Amoudi Steven D. Allison Lefteris Jason Ikuro Abe Anurag A. Agrawal Eric E. Alani Julian M. Allwood Anastasopoulos Stephen Tobias Abedon Ashutosh Agrawal Balbino Alarcón Eric J. Alm Hossain Anawar Moshe Abeles Rakesh Agrawal Qais Al-Awqati Benjamin A. Alman Elissar Andari Asa Abeliovich Jon Ågren Joseph Albanesi Ingvild Almas William R. L. Anderegg John Aber Alan Agresti Francis Albarede Steven C. Almo John M. Anderies Clara Abraham Jeremy J. Agresti Umberto Albarella Douglas Almond Mark L. Andermann John Abraham Jay J. Ague Silas D. Alben Uri Alon Bogi Andersen Daniel A. Abrams Fernan Agüero Frank Alber José M.