Effects of Host Abundance on Larch Budmoth Outbreaks in the European Alps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Health Improvement in Sub-Saharan
ARTIGO ARTICLE 37 Human health improvement in Sub-Saharan Africa through integrated management of arthropod transmitted diseases and natural resources Johann Baumgärtner 1 Markus Bieri 2 Giuseppe Buffoni 3 Mejoramiento de la salud humana en África Gianni Gilioli 4 al sur del Sahara mediante el manejo integrado Hiremagalur Gopalan 5 de enfermedades transmitidas por artrópodos Jürgen Greiling 1 y el manejo de recursos naturales Getachew Tikubet 1 Ingeborg Van Schayk 1 1 International Centre Abstract A concept of an ecosystem approach to human health improvement in Sub-Saharan of Insect Physiology and Africa is presented here. Three factors mainly affect the physical condition of the human body: Ecology (ICIPE), P.O. Box 30772, Nairobi, Kenya. the abiotic environment, vector-transmitted diseases, and natural resources. Our concept relies 2 Swiss Federal Institute on ecological principles embedded in a social context and identifies three sets of subsystems for of Technology (ETH), 8092 study and management: human disease subsystems, natural resource subsystems, and decision- Zurich, Switzerland. 3 Italian National Agency support subsystems. To control human diseases and to secure food from resource subsystems in- for New Technology, cluding livestock or crops, integrated preventive approaches are preferred over exclusively cura- Energy, and the Environment tive and sectorial approaches. Environmental sustainability – the basis for managing matter (ENEA), C.P.31619100, La Spezia, Italy. and water flows – contributes to a healthy human environment and constitutes the basis for so- 4 Dipartimento di cial sustainability. For planning and implementation of the human health improvement Agrochimica e Agrobiologia, scheme, participatory decision-support subsystems adapted to the local conditions need to be Università degli Studi di Reggio Calabria. -
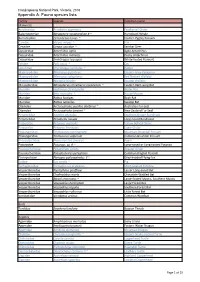
Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -
Home Pre-Fire Moth Species List by Species
Species present before fire - by species Scientific Name Common Name Family Abantiades aphenges Hepialidae Abantiades hyalinatus Mustard Ghost Moth Hepialidae Abantiades labyrinthicus Hepialidae Acanthodela erythrosema Oecophoridae Acantholena siccella Oecophoridae Acatapaustus leucospila Nolidae Achyra affinitalis Cotton Web Spinner Crambidae Aeolochroma mniaria Geometridae Ageletha hemiteles Oecophoridae Aglaosoma variegata Notodontidae Agriophara discobola Depressariidae Agrotis munda Brown Cutworm Noctuidae Alapadna pauropis Erebidae Alophosoma emmelopis Erebidae Amata nigriceps Erebidae Amelora demistis Pointed Cape Moth Geometridae Amelora sp. Cape Moths Geometridae Antasia flavicapitata Geometridae Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela repleta Anthelidae Anthela sp. Anthelidae Anthela varia Variable Anthelid Anthelidae Antipterna sp. Oecophoridae Ardozyga mesochra Gelechiidae Ardozyga sp. Gelechiidae Ardozyga xuthias Gelechiidae Arhodia lasiocamparia Pink Arhodia Geometridae Arrade destituta Erebidae Arrade leucocosmalis Erebidae Asthenoptycha iriodes Tortricidae Asura lydia Erebidae Azelina biplaga Geometridae Barea codrella Oecophoridae Calathusa basicunea Nolidae Calathusa hypotherma Nolidae Capusa graodes Geometridae Capusa sp. Geometridae Carposina sp. Carposinidae Casbia farinalis Geometridae Casbia sp. Geometridae Casbia tanaoctena Geometridae Catacometes phanozona Oecophoridae Catoryctis subparallela Xyloryctidae Cernia amyclaria Geometridae Chaetolopha oxyntis Geometridae Chelepteryx -

Foliage Insect Diversity in Dry Eucalypt Forests in Eastern Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 136, 2002 17 FOLIAGE INSECT DIVERSITY IN DRY EUCALYPT FORESTS IN EASTERN TASMANIA by H.J. Elliott, R. Bashford, S.J. Jarman and M.G. Neyland (with four tables, one text-figure and two appendices) ELLIOTT, H.]., BASHFORD, R., JARMAN,S.]' & NEYLAND, M.G., 2002 (3l:xii): Foliage insect diversity in dry eucalypt forests in eastern Tasmania. Papers and Proceedings ofthe Royal Society afTasmania 136: 17-34. ISSN 0080-4703. Forestry Tasmania, 79 Melville St., Hobart, Tasmania 7000, Australia. Species numbers and composition of the insect fauna occurring on trees and shrubs were studied in dry eucalypt forests in eastern Tasmania over nine years. In all, 1164 named and putative species representing 17 orders and 157 families were collected. The bulk of the species belonged to the orders Coleoptera (28%), Hymenoptera (25%), Hemiptera (18%), Lepidoptera (14%) and Diptera (10%). Of the species collected, 388 -- about one-third -- were identified at least to genus or species level. These included 21 named species not previously listed in the Tasmanian insect fauna and 90 undescribed species. A list of 22 host plants for 171 insect species was compiled from records of 132 insect species observed feeding during the study and from previous records ofinsect/host plant associations for 39 insect species found on the study plots. Most insects were feeding on eucalypts (127 insect species) and acacias (38 species). The most widely distributed and commonly collected species were several well-known pests ofeucalypts: Gonipterus scutellatus (Coleoptera: Curculionidae), Uraba lugens (Lepidoptera: N octuidae), Amorbus obscuricornis (Hemiptera: Coreidae), Chaetophyes compacta (Hemiptera: Machaerotidae) and Eriococcus coriaceous(Hemiptera: Eriococcidae). -

'Tasmanian Museum and Art Gallery's Expedition of Discovery I – the Flora
Papers and Proceedings of the Royal Society of Tasmania, Volume 153, 2019 5 TASMANIAN MUSEUM AND ART GALLERY’S EXPEDITION OF DISCOVERY I – THE FLORA AND FAUNA OF WIND SONG, LITTLE SWANPORT, TASMANIA by Matthew Baker, Simon Grove, Miguel de Salas, Catherine Byrne, Lyn Cave, Kevin Bonham, Kirrily Moore and Gintaras Kantvilas (with 15 plates, two tables and an appendix) Baker, M.L., Grove, S., de Salas, M.F., Byrne, C., Cave, L., Bonham, K., Moore, K. & Kantvilas, G. 2019 (14:xii): Tasmanian Museum and Art Gallery’s Expedition of Discovery I – The flora and fauna of Wind Song, Little Swanport, Tasmania. Papers and Proceedings of the Royal Society of Tasmania 153: 5–30. https://doi.org/10.26749/rstpp.153.5 ISSN 0080–4703. Tasmanian Museum and Art Gallery, GPO Box 1164, Hobart, Tasmania 7001, Australia (MLB*, SG, MFS, CB, LC, KB, KM, GK). *Author for correspondence. Email: [email protected] A flora and fauna survey was conducted at the east coast Tasmanian property Wind Song in 2017 as part of the Tasmanian Museum and Art Gallery’s ongoing research, collection-building and nature-discovery program. The survey recorded 885 taxa, primarily from the targeted groups of vascular plants, bryophytes, lichens, butterflies, moths, beetles, snails and slugs. Several of the taxa recorded, chiefly lichens and invertebrates, are new to science or new records for Tasmania. The survey provides a benchmark for further work and serves as an indicator of the biodiversity of a former farming property on Tasmania’s east coast. Key Words: species discovery, biodiversity, Tasmania, lichens, multidisciplinary survey. -
Diversity and Abundance of Lepidoptera and Coleoptera in Greenfleet
1 Australian Forestry 2 3 Diversity and abundance of Lepidoptera and Coleoptera in Greenfleet 4 reforestation plantings to offset carbon emissions: Proximity to remnants 5 will influence re-wilding of plantings 6 7 R. J. Forbesa, S. J. Watsona, E. O’Connorb, W. Wescottb and M. J. Steinbauera 8 9 aDepartment of Ecology, Environment and Evolution, La Trobe University, Melbourne, 10 Australia; bGreenfleet, 517 Flinders Lane, Melbourne, Australia 11 12 CONTACT Martin J. Steinbauer 13 email [email protected] 14 Address Department of Ecology, Environment and Evolution, La Trobe University, 15 Melbourne, VIC 3086 1 16 ABSTRACT 17 Mixed-species (floristically diverse) plantings of trees and shrubs in former agricultural 18 landscapes to offset (sequester) carbon emissions are a recent component of Australian 19 landscapes. Although their potential to mitigate biodiversity loss is recognised, this ecological 20 function has not been investigated, in particular with respect to insect diversity. Over two 21 summers, we used light trapping to sample Lepidoptera (moths) and Coleoptera (beetles) in 22 Greenfleet plantings in two distinct locations in Victoria (plantings of four ages per location) 23 as well as in nearby remnant forest and in pasture. At both locations, we found that plantings 24 had a greater abundance of Lepidoptera than remnants but that the abundance in plantings was 25 comparable to the abundance in pasture. The species richness of Lepidoptera in plantings did 26 not differ significantly from that in remnants but was significantly greater than that in pasture. 27 The abundance and species richness of Coleoptera in plantings was lower than in remnant 28 forests but higher than in pasture. -

Great Basin Naturalist Memoirs Volume 11 a Catalog of Scolytidae and Platypodidae Article 3 (Coleoptera), Part 1: Bibliography
Great Basin Naturalist Memoirs Volume 11 A Catalog of Scolytidae and Platypodidae Article 3 (Coleoptera), Part 1: Bibliography 1-1-1987 A–D Stephen L. Wood Life Science Museum and Department of Zoology, Brigham Young University, Provo, Utah 84602 Donald E. Bright Jr. Biosystematics Research Centre, Canada Department of Agriculture, Ottawa, Ontario, Canada 51A 0C6 Follow this and additional works at: https://scholarsarchive.byu.edu/gbnm Part of the Anatomy Commons, Botany Commons, Physiology Commons, and the Zoology Commons Recommended Citation Wood, Stephen L. and Bright, Donald E. Jr. (1987) "A–D," Great Basin Naturalist Memoirs: Vol. 11 , Article 3. Available at: https://scholarsarchive.byu.edu/gbnm/vol11/iss1/3 This Chapter is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist Memoirs by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. — J 987 Wood. BrichtCaTALOC Bin nookAim A IT A. A. 1924. A praga dos cafesaes paulistas. ( lorreio Agri- woods. Symposium on Southern I [ardwoods, Pro- cola 2(7):217-218, (en). ceedings 1971:80 89. (). 1927. *A. D, Neprite] kurovcu | Feir.de der Borkenkal'er] Abrahamson Lawrence Paul vnd Dau Melvin Noii P'riroda i znanie 20:27. (). his, Jii 1966a. Symbiotic interrelationship •A. L. 1899, Nagra iakttagelser angaende granbarkbor- tween microbes and ambrosia beetles: I The or rens fortplantningsoch lefnadssatt (Ips typogra- gans ol microbial transport and perpetuation of phies). Skogvaktaren. (). Xyloterinus politus. Entomological Society of *A. S. 1811. -
The Rise of Eversion Techniques in Lepidopteran Taxonomy (Insecta: Lepidoptera) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Mikkola, K. The rise of eversion techniques in lepidopteran taxonomy (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 35, núm. 139, septiembre, 2007, pp. 335-345 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45513910 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Nº 139 24/9/07 19:08 Página 335 SHILAP Revta. lepid., 35 (139), septiembre 2007: 335-345 CODEN: SRLPEF ISSN:0300-5267 The rise of eversion techniques in lepidopteran taxonomy (Insecta: Lepidoptera) K. Mikkola Abstract During the last decades of the 20th century, a new step to the traditional way of preparing lepidopteran micros- cope slides was widely adopted, the technique of inflating and fixing the soft parts of internal genitalia. The taxo- nomic resolution in revising problematic species groups, particularly allopatric relationships, improved considerably. At the same time, the so-called lock-and-key hypothesis has been revived, since usually the sexes show correspon- ding structural details in their internal genitalia. On the basis of intercontinental studies on the Noctuidae, it is con- sidered that the divergence of internal genitalia in pairs of sister species is based on genetic drift. The history of the eversion technique is revised. KEY WORDS: Insecta, Lepidoptera, internal genitalia, male vesica, female bursa, lock-and-key, microscopic slides. -

Mt Canobolas SCA AOBV Nomination – Medd & Bower - 2
Nomination of Mount Canobolas State Conservation Area as an Area of Outstanding Biodiversity Value 1Richard W. Medd PhD and 2Colin C. Bower PhD 1593 Cargo Road Orange, NSW 2800 [email protected] 2 PO Box 300, Orange, NSW 2800 Original Submitted May 2018 Revised with minor corrections July 2018 Citation: Medd RW and Bower CC (2018). Nomination of Mount Canobolas State Conservation Area as an Area of Outstanding Biodiversity Value. Submission to Office of Environment and Heritage, Unpublished 63pp Mt Canobolas SCA AOBV Nomination – Medd & Bower - 2 - Table of Contents Page Executive Summary ...................................................................................................... 3 1. Introduction ......................................................................................................... 7 2. Background ......................................................................................................... 7 3. Bioheritage .......................................................................................................... 8 3.1 Bryophytes 3.2 Vascular plants 3.3 Fungi 3.4 Vertebrates 3.5 Invertebrates 4. Threatened Ecological Communities .................................................................. 11 4.1 Xanthoparmelia Lichen Community 4.2 Tableland Basalt Forest Community 4.3 Tablelands Snow Gum Woodland Community 5. Threatened Species ............................................................................................ 12 5.1 Plants 5.2 Mammals 5.3 Birds 6. Endemic Species ............................................................................................... -

Form, Function and Evolution of the Mouthparts of Blood-Feeding Arthropodaq
Arthropod Structure & Development 41 (2012) 101e118 Contents lists available at SciVerse ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd Review Form, function and evolution of the mouthparts of blood-feeding Arthropodaq Harald W. Krenn a,*, Horst Aspöck b a Department of Evolutionary Biology, Faculty of Life Sciences, University Vienna, Althanstrasse 14, 1090 Vienna, Austria b Abteilung für Medizinische Parasitologie, Institut für Spezifische Prophylaxe und Tropenmedizin, Medizinische Universität Wien, Kinderspitalgasse 15, 1095 Vienna, Austria article info abstract Article history: This review compares the mouthparts and their modes of operation in blood-feeding Arthropoda which Received 1 September 2011 have medical relevance to humans. All possess piercing blood-sucking proboscides which exhibit thin Accepted 4 December 2011 stylet-shaped structures to puncture the host’s skin. The tips of the piercing structures are serrated to provide anchorage. Usually, the piercing organs are enveloped by a soft sheath-like part which is not Keywords: inserted. The piercing process includes either back and forth movements of the piercing structures, or Piercing mouthparts sideways cutting motions, or the apex of the proboscis bears teeth-like structures which execute drilling Feeding mechanism movements. Most piercing-proboscides have a food-canal which is separate from a salivary canal. The Blood-feeders Pathogens food-canal is functionally connected to a suction pump in the head that transports blood into the Insects alimentary tract. The salivary canal conducts saliva to the tip of the proboscis, from where it is discharged Mites into the host. Piercing blood-sucking proboscides evolved either from (1) generalized biting-chewing mouthparts, (2) from piercing mouthparts of predators, or plant sap or seed feeders, (3) from lapping or sponging mouthparts. -

Page 1 of 42
LIST OF TERRESTRIAL AND FRESHWATER INVERTEBRATES RECORDED FROM THE WHA The following list includes only formally named species, with the exception of un-named 'species' which are listed in Native Invertebrates which are Rare or Threatened in Tasmania (Inveretebrate Legend: Proportion of range in WHA: Part - <75% of State records in WHA ; Primary - >75% of State records in WHA; Entire - all State records in WHA; Distribution in WHA: Widespread - >4 widely dispersed records in WHA; Restricted - <5 widely dispersed records in WHA Tasmanian Terrestrial/ Proportion of Distribution GROUP/SPECIES Class Order Family Endemic Aquatic Habitat Range in WHA in WHA FLATWORMS Phylum: Platyhelminthes Artioposthia diemenensis (Dendy, 1894) Turbellaria Tricladida Geoplanidae (Caenoplaninae) yes terrestrial forest/rainforest/scrub part widespread Artioposthia dovei (Steel, 1901) Turbellaria Tricladida Geoplanidae (Caenoplaninae) yes terrestrial forest/rainforest part widespread Artioposthia mortoni (Dendy, 1894) Turbellaria Tricladida Geoplanidae (Caenoplaninae) yes terrestrial forest/rainforest/scrub part widespread Australoplana alba (Dendy, 1891) Turbellaria Tricladida Geoplanidae (Caenoplaninae) no terrestrial forest/rainforest part widespread Australoplana typhlops (Dendy, 1894) Turbellaria Tricladida Geoplanidae (Caenoplaninae) no terrestrial forest part widespread Fletchamia mediolineata (Dendy, 1891) Turbellaria Tricladida Geoplanidae (Caenoplaninae) no terrestrial forest/rainforest part restricted Fletchamia sugdeni (Dendy, 1891) Turbellaria Tricladida -

Die Autoren Der Taxa Der Rezenten Raphidiopteren (Insecta: Endopterygota)
© Österr. Ent. Ges. [ÖEG]/Austria; download unter www.biologiezentrum.at Entomologica Austriaca 21 9-152 Linz, 22.3.2014 Die Autoren der Taxa der rezenten Raphidiopteren (Insecta: Endopterygota) Horst ASPÖCK & Ulrike ASPÖCK Frau Professor Dr. Maria Matilde Principi, der Grande Dame der Neuropterologie, in Bewunderung, Verehrung und Dankbarkeit gewidmet. Sie ist, nunmehr 99 jährig, die älteste Neuropterologin, Vorbild mehrer Generationen, auch wir zählen uns zu ihren Schülern. Sie hat Standards von bleibender Gültigkeit in der wissenschaftlichen Erforschung und zeichnerischen Darstellung der Neuropterida gesetzt. Inhaltsübersicht Abstract .........................................................................................................10 1. Einführung.....................................................................................................10 2. Die 52 Autoren der Taxa der Artgruppe und der Gattungsgruppe der Raphidioptera ................................................................................................15 2.1. Vorbemerkung und Erläuterungen ................................................................15 2.2. Kurzbiographien und Listen der beschriebenen Taxa...................................19 3. Verzeichnis aller Publikationen mit Originalbeschreibungen von Taxa rezenter Raphidiopteren der Artgruppe und der Gattungsgruppe ...............124 4. Zusammenfassung.......................................................................................134 5. Dank ............................................................................................................134