Influence of the Little-Studied Sun's and Moon's Characteristics on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Barcoding of the Fire Ant Genus Solenopsis Westwood
Saudi Journal of Biological Sciences 27 (2020) 184–188 Contents lists available at ScienceDirect Saudi Journal of Biological Sciences journal homepage: www.sciencedirect.com Original article DNA barcoding of the fire ant genus Solenopsis Westwood (Hymenoptera: Formicidae) from the Riyadh region, the Kingdom of Saudi Arabia ⇑ Khawaja Ghulam Rasool a, , Mureed Husain a, Shehzad Salman a, Muhammad Tufail a,b, Sukirno Sukirno c, Abdulrahman S. Aldawood a a Department of Plant Protection, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia b Ghazi University, Dera Ghazi Khan, Punjab, Pakistan c Entomology Laboratory, Universitas Gadjah Mada, Indonesia article info abstract Article history: The ant genus Solenopsis Westwood, 1840 is the largest in Myrmicinae subfamily having almost 200 Received 29 April 2019 described species worldwide. They are commonly distributed in the tropics and temperate areas of the Revised 18 June 2019 world. Some invasive Solenopsis species are very dreadful. We have already reported a fire ant species, Accepted 30 June 2019 Solenopsis saudiensis Sharaf & Aldawood, 2011, identified using traditional morphometric approaches of Available online 2 July 2019 species identification. Present study was carried out to develop DNA Barcoding to identify Solenopsis sau- diensis and to elucidate genetic structure of the various S. saudiensis populations across their distribution Keywords: range in Riyadh, Saudi Arabia. The comparison of DNA barcodes showed no genetic diversity among six Fire ant populations and a queen from S. saudiensis analyzed from the Riyadh region. This genetic resemblance DNA barcoding Cytochrome C oxidase I probably reflects their adaptation toward a specific habitat, thus constituting a single and strong gene Biodiversity pool. -

Coleoptera and Lepidoptera (Insecta) Diversity in the Central Part of Sredna Gora Mountains (Bulgaria)
BULLETIN OF THE ENTOMOLOGICALENTOMOLOGICAL SOCIETY OF MALTAMALTA (2019) Vol. 10 : 75–95 DOI: 10.17387/BULLENTSOCMALTA.2019.09 Coleoptera and Lepidoptera (Insecta) diversity in the central part of Sredna Gora Mountains (Bulgaria) Rumyana KOSTOVA1*, Rostislav BEKCHIEV2 & Stoyan BESHKOV2 ABSTRACT.ABSTRACT. Despite the proximity of Sredna Gora Mountains to Sofia, the insect assemblages of this region are poorly studied. As a result of two studies carried out as a part of an Environmental Impact Assessment in the Natura 2000 Protected Areas: Sredna Gora and Popintsi, a rich diversity of insects was discovered, with 107 saproxylic and epigeobiont Coleoptera species and 355 Lepidoptera species recorded. This research was conducted during a short one-season field study in the surrounding areas of the town of Panagyurishte and Oborishte Village. Special attention was paid to protected species and their conservation status. Of the Coleoptera recorded, 22 species were of conservation significance. Forty-five Lepidoptera species of conservation importance were also recorded. KEY WORDS.WORDS. Saproxylic beetles, epigeobiont beetles, Macrolepidoptera, Natura 2000 INTRODUCTION INTRODUCTION The Sredna Gora Mountains are situated in the central part of Bulgaria, parallel to the Stara Planina Mountains The Sredna chain. Gora TheyMountains are insufficiently are situated instudied the central with partregard of toBulgaria, their invertebrate parallel to theassemblages. Stara Planina Mountains chain. They are insufficiently studied with regard to their invertebrate assemblages. There is lack of information about the beetles from Sredna Gora Mountains in the region of the Panagyurishte There is lack townof information and Oborishte about village. the beetles Most offrom the Srednaprevious Gora data Mountains is old and foundin the inregion catalogues, of the mentioningPanagyurishte the town mountain and Oborishte without distinct village. -

(Amsel, 1954) (Lepidoptera: Pyralidae, Phycitinae) – a New Species for the Croatian Pyraloid Moth Fauna, with an Updated Checklist
NAT. CROAT. VOL. 30 No 1 37–52 ZAGREB July 31, 2021 original scientific paper / izvorni znanstveni rad DOI 10.20302/NC.2021.30.4 PSOROSA MEDITERRANELLA (AMSEL, 1954) (LEPIDOPTERA: PYRALIDAE, PHYCITINAE) – A NEW SPECIES FOR THE CROATIAN PYRALOID MOTH FAUNA, WITH AN UPDATED CHECKLIST DANIJELA GUMHALTER Azuritweg 2, 70619 Stuttgart, Germany (e-mail: [email protected]) Gumhalter, D.: Psorosa mediterranella (Amsel, 1954) (Lepidoptera: Pyralidae, Phycitinae) – a new species for the Croatian pyraloid moth fauna, with an updated checklist. Nat. Croat., Vol. 30, No. 1, 37–52, 2021, Zagreb. From 2016 to 2020 numerous surveys were undertaken to improve the knowledge of the pyraloid moth fauna of Biokovo Nature Park. On August 27th, 2020 one specimen of Psorosa mediterranella (Amsel, 1954) from the family Pyralidae was collected on a small meadow (985 m a.s.l.) on Mt Biok- ovo. In this paper, the first data about the occurrence of this species in Croatia are presented. The previ- ous mention in the literature for Croatia was considered to be a misidentification of the past and has thus not been included in the checklist of Croatian pyraloid moth species. P. mediterranella was recorded for the first time in Croatia in recent investigations and, after other additions to the checklist have been counted, is the 396th species in the Croatian pyraloid moth fauna. An overview of the overall pyraloid moth fauna of Croatia is given in the updated species list. Keywords: Psorosa mediterranella, Pyraloidea, Pyralidae, fauna, Biokovo, Croatia Gumhalter, D.: Psorosa mediterranella (Amsel, 1954) (Lepidoptera: Pyralidae, Phycitinae) – nova vrsta u hrvatskoj fauni Pyraloidea, s nadopunjenim popisom vrsta. -

Draft Pest Categorisation of Organisms Associated with Washed Ware Potatoes (Solanum Tuberosum) Imported from Other Australian States and Territories
Nucleorhabdovirus Draft pest categorisation of organisms associated with washed ware potatoes (Solanum tuberosum) imported from other Australian states and territories This page is intentionally left blank Contributing authors Bennington JMA Research Officer – Biosecurity and Regulation, Plant Biosecurity Hammond NE Research Officer – Biosecurity and Regulation, Plant Biosecurity Poole MC Research Officer – Biosecurity and Regulation, Plant Biosecurity Shan F Research Officer – Biosecurity and Regulation, Plant Biosecurity Wood CE Technical Officer – Biosecurity and Regulation, Plant Biosecurity Department of Agriculture and Food, Western Australia, December 2016 Document citation DAFWA 2016, Draft pest categorisation of organisms associated with washed ware potatoes (Solanum tuberosum) imported from other Australian states and territories. Department of Agriculture and Food, Western Australia, South Perth. Copyright© Western Australian Agriculture Authority, 2016 Western Australian Government materials, including website pages, documents and online graphics, audio and video are protected by copyright law. Copyright of materials created by or for the Department of Agriculture and Food resides with the Western Australian Agriculture Authority established under the Biosecurity and Agriculture Management Act 2007. Apart from any fair dealing for the purposes of private study, research, criticism or review, as permitted under the provisions of the Copyright Act 1968, no part may be reproduced or reused for any commercial purposes whatsoever -

Cómo Citar El Artículo Número Completo Más Información Del
Acta zoológica mexicana ISSN: 0065-1737 ISSN: 2448-8445 Instituto de Ecología A.C. Molina-Martínez, Arcángel; Aragón-García, Agustín; Pérez-Torres, Betzabeth Cecilia Nuevos registros de palomillas (Sphingidae y Erebidae) para el estado de Puebla Acta zoológica mexicana, vol. 34, e3412102, 2018 Instituto de Ecología A.C. DOI: 10.21829/azm.2018.3412102 Disponible en: http://www.redalyc.org/articulo.oa?id=57560238021 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto e ISSN 2448-8445 (2018) Volumen 34, 1–4 ISSN 0065-1737 elocation- id: e3412102 https://doi.org/10.21829/azm.2018.3412102 Nota científica (Short communication) NUEVOS REGISTROS DE PALOMILLAS (SPHINGIDAE Y EREBIDAE) PARA EL ESTADO DE PUEBLA NEW RECORDS OF MOTHS (SPHINGIDAE AND EREBIDAE) FOR THE STATE OF PUEBLA 1 1 ARCÁNGEL MOLINA-MARTÍNEZ *, AGUSTÍN ARAGÓN-GARCÍA & BETZABETH CECILIA PÉREZ- TORRES1 1Centro de Agroecología, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla. Edificio VAL 1, Km 1.7 Carretera a San Baltazar Tetela, San Pedro Zacachimalpa, Puebla, Puebla. C.P. 72960 México. <[email protected]>; <[email protected]>; <[email protected]> *Autor de correspondencia: <[email protected]> Recibido: 14/04/2016; aceptado: 08/02/2018; publicado en línea: 22/10/2018 Editor responsable: Arturo Bonet Molina-Martínez, A., Aragón-García, A., Pérez-Torres, B. C. (2018) Nuevos registros de palomillas (Sphingidae y Erebidae) para el estado de Puebla. -
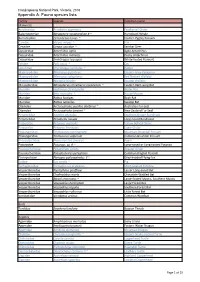
Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

Check List of Noctuid Moths (Lepidoptera: Noctuidae And
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (2), стор. 87–97, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (2), pp. 87–97, 2016 ARTICLE UDC 595.786 CHECK LIST OF NOCTUID MOTHS (LEPIDOPTERA: NOCTUIDAE AND EREBIDAE EXCLUDING LYMANTRIINAE AND ARCTIINAE) FROM THE SAUR MOUNTAINS (EAST KAZAKHSTAN AND NORTH-EAST CHINA) A.V. Volynkin1, 2, S.V. Titov3, M. Černila4 1 Altai State University, South Siberian Botanical Garden, Lenina pr. 61, Barnaul, 656049, Russia. E-mail: [email protected] 2 Tomsk State University, Laboratory of Biodiversity and Ecology, Lenina pr. 36, 634050, Tomsk, Russia 3 The Research Centre for Environmental ‘Monitoring’, S. Toraighyrov Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan. E-mail: [email protected] 4 The Slovenian Museum of Natural History, Prešernova 20, SI-1001, Ljubljana, Slovenia. E-mail: [email protected] The paper contains data on the fauna of the Lepidoptera families Erebidae (excluding subfamilies Lymantriinae and Arctiinae) and Noctuidae of the Saur Mountains (East Kazakhstan). The check list includes 216 species. The map of collecting localities is presented. Key words: Lepidoptera, Noctuidae, Erebidae, Asia, Kazakhstan, Saur, fauna. INTRODUCTION The fauna of noctuoid moths (the families Erebidae and Noctuidae) of Kazakhstan is still poorly studied. Only the fauna of West Kazakhstan has been studied satisfactorily (Gorbunov 2011). On the faunas of other parts of the country, only fragmentary data are published (Lederer, 1853; 1855; Aibasov & Zhdanko 1982; Hacker & Peks 1990; Lehmann et al. 1998; Benedek & Bálint 2009; 2013; Korb 2013). In contrast to the West Kazakhstan, the fauna of noctuid moths of East Kazakhstan was studied inadequately. -

Identification of Insects, Spiders and Mites in Vegetable Crops: Workshop Manual Loopers Chrysodeixis Spp
Important pests in vegetable crops This section gives descriptions of common pests found in Australian vegetable crops. They are listed according to the insect order they belong to. Moths and butterflies (Lepidoptera) Heliothis (corn earworm, tomato budworm, native budworm) Helicoverpa spp. Heliothis larvae feed as leaf eaters and bud and fruit borers on a wide range of plants, including many weeds. Adults lay round, domed, ribbed eggs that are cream when newly laid, turning brownish (‘brown ring’ stage) as they mature. Larvae grow up to 40 mm long and vary in colour from green through yellow and brown to almost black, with a pale stripe down each side. The moths have a wing span of 35–45 mm. Heliothis moth at rest These are some examples of heliothis damage to vegetable crops: • On lettuce and brassicas, larvae feed on the outer leaves or tunnel into the heart of the plant. • In tomato crops, eggs are laid on the leaves, flowers and fruit. Young larvae burrow into flowers causing them to fall and they cause pinhole damage to very young tomato fruit. Older larvae burrow into the fruit, creating holes and encouraging rots to develop. • In capsicum, larvae feed on the fruit and the seed inside the fruit. Eggs are laid mainly on leaves, but also on flowers and buds. Heliothis eggs on tomato shoot • In sweet corn, eggs are laid on the silks and leaves. Larvae feed on the developing grains on the cob, sometimes on the leaves and often inside the tip of the corn cob. • In green beans and peas, the larvae feed on the flowers, pods and developing seed inside the pods. -

Assessing the Invertebrate Fauna Trajectories in Remediation Sites of Winstone Aggregates Hunua Quarry in Auckland
ISSN: 1179-7738 ISBN: 978-0-86476-417-1 Lincoln University Wildlife Management Report No. 59 Assessing the invertebrate fauna trajectories in remediation sites of Winstone Aggregates Hunua quarry in Auckland by Kate Curtis1, Mike Bowie1, Keith Barber2, Stephane Boyer3 , John Marris4 & Brian Patrick5 1Department of Ecology, Lincoln University, PO Box 85084, Lincoln 7647 2Winstone Aggregates, Hunua Gorge Road, Red Hill 2110, Auckland 3Department of Nature Sciences, Unitec Institute of Technology, PO Box 92025, Auckland 1142. 4Bio-Protection Research Centre, Lincoln University, PO Box 85084, Lincoln 7647. 5Consultant Ecologist, Wildlands, PO Box 33499, Christchurch. Prepared for: Winstone Aggregates April 2016 Table of Contents Abstract……………………………………………………………………………………....................... 2 Introduction…………………………………………………………………………………………………… 2 Methodology…………………………………………………………………………………………………. 4 Results…………………………………………………………………………………………………………… 8 Discussion……………………………………………………………………………………………………. 31 Conclusion…………………………………………………………………………………………………… 37 Recommendations………………………………………………………………………………………. 38 Acknowlegdements……………………………………………………………………………………… 38 References…………………………………………………………………………………………………… 39 Appendix……………………………………………………………………………………………………… 43 1 Abstract This study monitored the invertebrates in restoration plantings in the Winstone Aggregates Hunua Quarry. This was to assess the re-establishment of invertebrates in the restoration planting sites and compare them with unplanted control and mature sites. This study follows on from -

The Noctuidae (Lepidoptera) of the Daghestan Republic (Russia)
The Noctuidae (Lepidoptera) of the Daghestan Republic (Russia) Poltavsky Alexander Nikolaevitch & Ilyina Elena Vjatcheslavovna Abstract. In this paper the complete list of Noctuidae currently known from Daghestan, the largest republic in the North Caucasus, is given. The list comprises 343 species and includes original data of the authors, records from the two main national collections in Russia, and some data from a few publications. Noctuidae were recorded from 37 localities in Daghestan, situated in the five natural zones of the country. The time interval of the faunistic studies spreads through the main part of the 20th Century: from 1926 to 2000. Samenvatting. De Noctuidae (Lepidoptera) van de Republiek Daghestan (Rusland) Dit artikel bevat de volledige lijst van de 343 soorten Noctuidae die tot op heden bekend zijn uit Daghestan, de grootste republiek in de Noord-Kaukasus. De lijst werd samengesteld met persoonlijke waarnemingen van de auteurs, gegevens uit de twee belangrijkste verzamelingen in Rusland en enkele gepubliceerde gegevens. Noctuidae werden op 37 plaatsen verzameld in Daghestan, gelegen in de 5 natuurlijke gebieden van het land. De waarnemingen stammen uit een grote tijdspanne in de 20ste eeuw: van 1926 tot 2000. Résumé. Les Noctuidés (Lepidoptera) de la République du Daghestan (Russie) Cet article contient la liste complète des 343 espèces de Noctuidae qui sont connues du Daghestan, la république la plus grande du Nord-Caucase. La liste a été compilée avec les observations personnelles des auteurs, les données des deux plus grandes collections de Russie et quelques citations dans la littérature. Des Noctuidae furent capturés dans 37 localités différentes, situées dans les 5 zones naturelles du pays. -

The Little Things That Run the City How Do Melbourne’S Green Spaces Support Insect Biodiversity and Promote Ecosystem Health?
The Little Things that Run the City How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Luis Mata, Christopher D. Ives, Georgia E. Garrard, Ascelin Gordon, Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk, Kirsten Parris and Sarah A. Bekessy The Little Things that Run the City – How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Report prepared for the City of Melbourne, November 2015 Coordinating authors Luis Mata Christopher D. Ives Georgia E. Garrard Ascelin Gordon Sarah Bekessy Interdisciplinary Conservation Science Research Group Centre for Urban Research School of Global, Urban and Social Studies RMIT University 124 La Trobe Street Melbourne 3000 Contributing authors Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk and Kirsten Parris. Cover artwork by Kate Cranney ‘Melbourne in a Minute Scavenger’ (Ink and paper on paper, 2015) This artwork is a little tribute to a minute beetle. We found the brown minute scavenger beetle (Corticaria sp.) at so many survey plots for the Little Things that Run the City project that we dubbed the species ‘Old Faithful’. I’ve recreated the map of the City of Melbourne within the beetle’s body. Can you trace the outline of Port Phillip Bay? Can you recognise the shape of your suburb? Next time you’re walking in a park or garden in the City of Melbourne, keep a keen eye out for this ubiquitous little beetle. -

Landcorp Denmark East Development Precinct Flora and Fauna Survey
LandCorp Denmark East Development Precinct Flora and Fauna Survey October 2016 Executive summary Introduction Through the Royalties for Regions “Growing our South” initiative, the Shire of Denmark has received funding to provide a second crossing of the Denmark River, to upgrade approximately 6.5 km of local roads and to support the delivery of an industrial estate adjacent to McIntosh Road. GHD Pty Ltd (GHD) was commissioned by LandCorp to undertake a biological assessment of the project survey area. The purpose of the assessment was to identify and describe flora, vegetation and fauna within the survey area. The outcomes of the assessment will be used in the environmental assessment and approvals process and will identify the possible need for, and scope of, further field investigations will inform environmental impact assessment of the road upgrades. The survey area is approximately 68.5 ha in area and includes a broad area of land between Scotsdale Road and the Denmark River and the road reserve and adjacent land along East River Road and McIntosh Road between the Denmark Mt Barker Road and South Western Highway. A 200 m section north and south along the Denmark Mt Barker Road from East River Road was also surveyed. The biological assessment involved a desktop review and three separate field surveys, including a winter flora and fauna survey, spring flora and fauna survey and spring nocturnal fauna survey. Fauna surveys also included the use of movement sensitive cameras in key locations. Key biological aspects The key biological aspects and constraints identified for the survey area are summarised in the following table.