United States Patent Office Patented Oct
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Agrimer™ Polyvinylpyyrolidone (PVP)
agrimer ™ polyvinylpyyrolidone (PVP) binder, dispersant rheology, modifier, film former, complexing agent Agrimer™ polyvinylpyrrolidone (PVP) this brochure is divided into two main segments suggested applications General properties and uses 2-10 ¢ complexing agent Agricultural case studies 10 ¢ stabilizers / co-dispersants These case studies highlight the uses of Agrimer™ ¢ binders in dry / wet granulation and extrusion (dry compaction / fluidized-bed spray drying process) polymers in seed coatings, granule and tablet binders and as dispersants. ¢ film-forming agents / binders in seed coatings, dips and pour-ons general properties and uses ¢ biological stabilization ¢ water binding / anti-transpiration properties Agrimer™ PVP products are linear, non-ionic polymers that are soluble in water and many organic solvents. ¢ solubility enhancers via co-precipitation or They are pH stable, and have adhesive, cohesive thermal extrusion and binding properties. The unique ability to adsorb ¢ dye-binding agent on a host of active ingredients makes Agrimer™ PVP regulatory status homopolymers preferred co-dispersants in many The Agrimer™ PVP products listed in this brochure are formulations. Agrimer™ homopolymers have a high exempt from the requirement of a tolerance under glass transition temperature. 40 CFR 180.960. Lower molecular weight (Mw) Agrimer™ polymers (Agrimer™ 15 and Agrimer™ 30) are suitable for physical and chemical properties applications where dusting is a concern, such as The Agrimer™ polymers, a family of homopolymers of seed coatings and agglomeration. Higher Mw polyvinylpyrrolidone, are available in different viscosity Agrimer™ polymers (Agrimer™ 90 and Agrimer™ 120) can grades, ranging from very low to very high molecular build formulation viscosity faster and provide excellent weight. This range, coupled with their solubility in binding and film forming properties. -

Chemical Compatibility Chart X
Chemical Compatibility Chart Below is a chart adapted from the CRC Laboratory Handbook, which groups various chemicals in to 23 groups with examples and incompatible chemical groups. This chart is by no means complete but it will aid in making decisions about storage. For more complete information please refer to the MSDS for the specific chemical. Examples of each group can be found on the next pages. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Monomers Polymerizable Esters Alcohols, Glycols, Glycol Ether Amines and Alkanolamines Halogenated Compounds Aldehydes Acetaldehyde Saturated Hydrocar Aromatic Hydrocarbons Acid Anhydrides Alkylene Oxides Inorganic Acids Petrolium Oils Organic Acids Cyanohydrins Phosphorus Ammonia Group Halogens Ketones Caustics Phenols Nitriles Olefins Ethers Number/Chemical Esters Type bons Inorganic 1 x x x x x x x x x x x x x x x x x Acids 2 Organic Acids x x x x x x x x x x 3 Caustics x x x x x x x x x x x x x Amines and 4 x x x x x x x x x x x x Alkanolamines Halogenated 5 x x x x x x Compounds Alcohols, 6 Glycols, Glycol x x x x x x Ether Aldehydes 7 x x x x x x x x x x x x Acetaldehyde 8 Ketones x x x x x x Saturated 9 x Hydrocarbons Aromatic 10 x x Hydrocarbons 11 Olefins x x x 12 Petrolium Oils x 13 Esters x x x x x Monomers 14 Polymerizable x x x x x x x x x x x x Esters 15 Phenols x x x x x x x Alkylene 16 x x x x x x x x x x x x Oxides 17 Cyanohydrins x x x x x x x x x 18 Nitriles x x x x x x 19 Ammonia x x x x x x x x x x x 20 Halogens x x x x x x x x x x x x x x 21 Ethers x x x 22 Phosphorus x x x x Acid 23 x x x x x x x x x x Anhydrides X - Indicates chemicals that are incompatible and should not be stored together. -
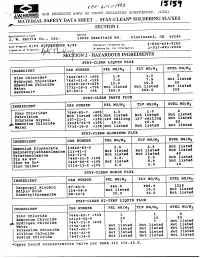
Section 2 - Hazardous Ingredients
rev- OUR PRODUCTS HAVE NO OZONE DEPLETING SUBSTANCES. (ODS) SAFETY DATA SHEET - STAY-CLEAN* SOLDERING FLUXES SECTION 1 Address Manufacturer's Name Cincinnati, OH 45242 j. w. Harris Co., Inc. 10930 Deerfield Rd. 1-800-424-9300 ES 8/92 Emergency Telephone No. Date Prepared 6/93 SUP 1-513-891-2000 Telephone Mo. for Information Signature of Preparer: Section 2 - hazardous ingredients STAY-CLEAN LIQUID FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 7.5 Not listed Hydrogen chloride* 7647-01-0 <15% 7.0 12125-02-9<25% 10.0 10.0 20 Ammonium chloride Not listed 7732-18-5 <70% Not listed Not listed Water 260.0 262.0 325 Methanol* 67-56-1 <5% STAY-CLEAN PASTE FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 Not listed Not listed Not listed Petrolatum Not listed <80% 127 ceiling Not listed Ethylene Glycol 107-21-1 <15% 125 ceiling 10.0 20 Ammonium Chloride 12125-02-9 <10% 10.0 Not listed Not listed Not listed Water 7732-18-5 <10% STAY-CLEAN ALUMINUM FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 2.5 Not listed Ammonium Fluoborate 13826-83-0 2.5 Not listed Not listed Aminoethylethanolamine 111-41-1 Not listed Not listed 60 Triethanolamine 102-71-6 Not listed 2.0 Not listed Tin as Sn* 7440-31-5 <10% 2.0. -

Chemical Compatibility Chart
Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride Oleum Methylene chloride Phosphoric acid Monochlorodifluoromethane Sulfuric acid Perchloroethylene Propylene dichloride Group 2: Organic Acids 1,2,4-Trichlorobenzene Acetic acid 1,1,1-Trichloroethane Butyric acid (n-) Trichloroethylene Formic acid Trichlorofluoromethane Propionic acid Rosin Oil Group 6: Alcohols, Glycols and Glycol Ethers Tall oil Allyl alcohol Amyl alcohol Group 3: Caustics 1,4-Butanediol Caustic potash solution Butyl alcohol (iso, n, sec, tert) Caustic soda solution Butylene -

Fr9de98b.Pdf
Federal Register / Vol. 63, No. 236 / Wednesday, December 9, 1998 / Proposed Rules 68037 (ii) The required mass removal is downstream of the point of Ci=Concentration of VOC at the point of calculated by summing the required determination with adjustment for determination, parts per million by mass removal for all wastewater streams concentration change made according to weight. combined for treatment when § 60.782(b)(6) of this subpart. Fri=Fraction removal value of a VOC. complying with § 60.779(g)(1)(i) or (g)(2) Concentration measurements to Follow the procedures in § 60.778 of this subpart. determine AMR shall be taken at the of this subpart to develop a stream- (5) The AMR calculation procedure inlet and outlet to the treatment process specific list of VOC. Follow the for non-combustion treatment processes and as provided in paragraph (a)(7) of procedures in appendix J of this including closed biological treatment this section for a series of treatment part to determine Fr values. processes. The AMR shall be calculated processes. Wastewater samples shall be 10 9=Conversion factor, mg/kg * l/m 3. as follows: collected using sampling procedures (4) The required mass removal is which minimize loss of organic calculated by adding together the = − AMR() QMWa QMW b () Eqn WW10 compounds during sample collection required mass removal for each Group 1 Where: and analysis and maintain sample wastewater stream to be combined for AMR=Actual mass removal of VOC integrity per § 60.782(b)(5)(ii) of this treatment. achieved by treatment process or subpart. The method shall be an (5) Actual mass removal calculation series of treatment processes, analytical method for wastewater which procedure for open or closed aerobic kilograms per hour. -

Table 1 to Subpart Yyy—List of Socmi Chemicals
TABLE 1 TO SUBPART YYY—LIST OF SOCMI CHEMICALS Chemical namea CAS No.b (1,1,2-) Trichloro (1,2,2-) trifluoroethane 76131 (2-Ethylhexyl) amine 104756 1,4-Dichlorobutene 110576 1-Butene 106989 1-Methyl-2-pyrrolidone 872504 1-Naphthyl-N-methylcarbamate 3071327 1-Phenyl ethyl hydroperoxide 25167673 2-Butene 110656 2-Butyne-1,4-diol 126998 2-Chloro-4-(ethylamino)-6-(isopropylamino)-S-triazine 1912249 2-Ethylhexanol (2-ethyl-1-hexanol) 104767 2-Hexenedinitrile 13042029 3,4-Dichloro-1-butene 64037543 3-Hexenedintrile 1119853 3-Pentenenitrile 4635874 6-Ethyl-1,2,3,4-tetrahydro-9,10-antracenedione 15547178 Acenaphthene 83329 Acetal (1,1-diethoxy-ethane) 105577 Acetaldehyde 75070 Acetaldol (3-hydroxy-butanal) 107891 Acetamide 60355 Acetanilide 103844 Acetic anhydride 108247 Acetic acid 64197 Acetoacetanilide 102012 Acetone cyanohydrin 75865 Acetone 67641 Acetonitrile 75058 Acetophenone 98862 Acetyl chloride 75365 Acetylene tetrabromide (1,1,2,2-tetrabromomethane) 79276 Acetylene 74862 Acrolein 107028 Acrylamide 79061 Acrylic acid 79107 Acrylonitrile 107131 Adipic acid 124049 Adiponitrile 111693 Alcohols, C-11 or higher, mixtures Alcohols, C-11 or lower, mixtures Alizarin 72480 Alkyl naphthalenes Alkyl naphthalene sulfonates Alkyl anthraquinones Allyl cyanide 109751 Allyl chloride 107051 Allyl bromide 106956 TABLE 1 TO SUBPART YYY—LIST OF SOCMI CHEMICALS - Continued Chemical namea CAS No.b Allyl alcohol 107186 Aluminum acetate 7360443 Aluminum formates Aminobenzoic acid (p-) 1321115 Aminoethylethanolamine 111411 Aminophenol sulfonic acid Aminophenol -

Incompatible Chemicals Focus Sheet
INCOMPATIBLE CHEMICALS CHEMICAL COMPATIBILITY CHART Below is a chart adapted from the CRC Laboratory Handbook which groups various chemicals into 23 groups with examples and incompatible chemical groups. This chart is by no means complete but it will aid in making decisions about storage. For more complete information please refer to the MSDS/SDS for the specific chemical. Group Name Example Incompatible Groups Hydrochloric acid Hydrofluoric acid Hydrogen chloride 1 Inorganic Acids Hydrogen fluoride 2,3,4,5,6,7,8,10,13,14,16,17,18,19,21,22,23 Nitric acid Sulfuric acid Phosphoric acid Acetic acid Butyric acid 2 Organic acids 1,3,4,7,14,16,17,18,19,22 Formic acid Propionic acid Sodium hydroxide 3 Caustics 1,2,6,7,8,13,14,15,16,17,18,20,23 Ammonium hydroxide solution Aminoethylethanolamine Aniline Diethanolamine Diethylamine Dimethylamine Ethylenediamine 4 Amines and Alkanolamines 1,2,5,7,8,13,14,15,16,17,18,23 2-Methyl-5-ethylpyridine Monoethanolamine Pyridine Triethanolamine Triethylamine Triethylenetetramine Allyl chloride Carbon tetrachloride Chlorobenzene Chloroform Methylene chloride 5 Halogenated Compounds Monochlorodifluoromethane 1,3,4,11,14,17 1,2,4-Trichlorobenzene 1,1,1-Trichloroethane Trichloroethylene Trichlorofluoromethane EH&S Building & Fire Safety | June 2017 │www.ehs.washington.edu | 206.543.7262 | [email protected] | Page 1 Group Name Example Incompatible Groups 1,4-Butanediol Butanol (iso, n, sec, tert) Diethylene glycol Ethyl alcohol Alcohols Ethyl butanol 6 Glycols Ethylene glycol 1,7,14,16,20,23 Glycol Ether Furfuryl -

40 CFR Ch. I (7–1–97 Edition) § 60.488
§ 60.488 40 CFR Ch. I (7±1±97 Edition) paragraphs (a) through (c) of this sec- CAS No. a Chemical tion, provided that they comply with 75±05±8 ............. Acetonitrile. the requirements established by the 98±86±2 ............. Acetophenone. State. 75±36±5 ............. Acetyl chloride. 74±86±2 ............. Acetylene. [48 FR 48335, Oct. 18, 1983, as amended at 49 107±02±8 ........... Acrolein. FR 22608, May 30, 1984] 79±06±1 ............. Acrylamide. 79±10±7 ............. Acrylic acid. § 60.488 Reconstruction. 107±13±1 ........... Acrylonitrile. 124±04±9 ........... Adipic acid. For the purposes of this subpart: 111±69±3 ........... Adiponitrile. (a) The cost of the following fre- (b) ....................... Alkyl naphthalenes. 107±18±6 ........... Allyl alcohol. quently replaced components of the fa- 107±05±1 ........... Allyl chloride. cility shall not be considered in cal- 1321±11±5 ......... Aminobenzoic acid. culating either the ``fixed capital cost 111±41±1 ........... Aminoethylethanolamine. of the new components'' or the ``fixed 123±30±8 ........... p-Aminophenol. 628±63±7, 123± Amyl acetates. capital costs that would be required to 92±2. construct a comparable new facility'' 71±41±0 c ........... Amyl alcohols. under § 60.15: pump seals, nuts and 110±58±7 ........... Amyl amine. 543±59±9 ........... Amyl chloride. bolts, rupture disks, and packings. 110±66±7 c ......... Amyl mercaptans. (b) Under § 60.15, the ``fixed capital 1322±06±1 ......... Amyl phenol. cost of new components'' includes the 62±53±3 ............. Aniline. 142±04±1 ........... Aniline hydrochloride. fixed capital cost of all depreciable 29191±52±4 ....... Anisidine. components (except components speci- 100±66±3 ........... Anisole. fied in § 60.488 (a)) which are or will be 118±92±3 .......... -

Product and Product Group Discharges Subject to Effluent Limitations and Standards for Organic Chemicals, Plastics, and Syntheti
PRODUCT AND PRODUCT GROUP DISCHARGES SUBJECT TO EFFLUENT LIMITATIONS AND STANDARDS for the ORGANIC CHEMICALS, PLASTICS, AND SYNTHETIC FIBERS POINT SOURCE CATEGORY - 40 CFR 414 April 2005 Office of Water U.S. Environmental Protection Agency 1200 Pennsylvania Ave., N.W. Washington, D.C. 20460 Table of Contents Section Page Introduction. 1 1 Summary of the 40 CFR 414 . .2 1.1 Regulated Parameters. 3 1.2 Requirements for Direct and Indirect Dischargers. 3 2. Identifying and Classifying Products Whose Production May be Subject to the OCPSF Regulation. 5 2.1 The SIC Manual and Codes . 5 2.2 Industrial Categories Applicable to Chemicals and Allied Products. .6 2.3 Part 414 Applicability to Production of Chemicals and Chemical Products. .6 2.4 Applicability of Wastewater from On-Site Auxiliary Operations. .8 2.5 General Discussion of OCPSF-Related Products Whose Manufacture May Not Be Regulated by Part 414 . .8 2.5.1 Products Classified and Previously Reported under Specific SIC Codes That Are Not Subject to Part 414 . .8 2.5.2 Products Listed in Part 414 That Are Regulated by Another Industrial Category Are Not Subject to Part 414 in Certain Circumstances . .10 2.5.2.1 Organic Chemicals Regulated by the Iron and Steel Category (40 CFR 420) . 10 2.5.2.2 Organic Chemicals Regulated by the Pesticides Chemicals Category (40 CFR 455). 11 2.5.2.3 Organic Chemicals Regulated by the Pharmaceutical Category. 11 2.5.2.4 Products Regulated by the Plastics Molding & Forming Category (40 CFR 463). 12 2.5.2.5 Organic Chemicals Regulated by the Soap and Detergent Category (40 CFR 417). -
Incompatibilities of Concentrated Nitric Acid: Never Mix Concentrated Nitric
Incompatibilities of concentrated nitric acid: Never mix concentrated nitric acid and organics, such as acetone, unless you are following a respectable procedure and use a blast shield and proper precautions. Never store mixtures of concentrated acids, particularly nitric acid, and organic or inorganic waste components; immediately dilute any mixtures generated from concentrated acids by slow addition to ice or water in an open plastic container or a plastic bottle behind a shield or hood sash. Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride -

(BSSL) | V10.0 | July 01, 2019 © Bluesign Technologies Ag | 2 | 73
bluesign® system substances list (BSSL) Consumer safety limits Version 10.0 | July 1, 2019 Content 1 Introduction 4 2 Definitions 4 2.1 Accessory 4 2.2 Article 4 2.3 BSSL 4 2.4 CAS 4 2.5 Chemical substance 4 2.6 Component 4 2.7 Detection limit (DL) 4 2.8 Limit value 4 2.9 Mixture 4 2.10 Monitoring 5 2.11 Several 5 2.12 Traces 5 2.13 Usage ban 5 2.14 Usage range 5 3 Testing methods 6 4 Scope and validity 7 4.1 Application 7 4.2 Validity 7 5 Consumer safety limits and usage restrictions 8 5.1 Consumer safety limits 8 5.1.1 pH 8 5.1.2 Odor 8 5.1.3 Sensitizing disperse dyes 8 5.1.4 Textiles dyed with disperse or metal complex dyes 8 5.1.5 Color fastness to saliva and perspiration 8 5.1.6 Restrictions and bans for chemical substances 8 Aldehydes 9 Alkylphenols and Alkylphenolethoxylates 10 Amines 11 Arylamines (including corresponding salts) 12 Asbestos 14 Biocides 15 Chlorinated Benzenes and Toluenes 17 Chlorinated Phenols 18 Colorants 20 Dioxins and Furans 23 Flame Retardants 24 Fluorinated Substances 26 Glycols 29 Greenhouse Gases, fluorinated 30 Halogenated Biphenyls, halogenated Terphenyls and halogenated Naphthalenes 30 Halogenated Diarylalkanes 31 Isocyanates 32 bluesign® system substances list (BSSL) | v10.0 | July 01, 2019 © bluesign technologies ag | www.bluesign.com 2 | 73 Metals 33 Monomers 38 Nitrosamines 40 Other Chemical Substances 41 Ozone Depleting Substances 46 Pesticides 46 Plasticizers 47 Polyaromatic Hydrocarbons (PAHs) 49 Polymers 50 Solvents 51 Tin-organic Compounds 55 UV stabilizers 56 5.2 Substances with usage restrictions but no consumer safety limits 57 Annex I Compilation of single substances 59 Annex II Usage Ranges 71 bluesign® system substances list (BSSL) | v10.0 | July 01, 2019 © bluesign technologies ag | www.bluesign.com 3 | 73 1 Introduction The document specifies the limits for chemical substances in articles. -

Department of Homeland Security
Vol. 80 Thursday, No. 204 October 22, 2015 Part IV Department of Homeland Security Coast Guard 46 CFR Parts 30, 150, and 153 2013 Liquid Chemical Categorization Updates; Proposed Rule VerDate Sep<11>2014 20:20 Oct 21, 2015 Jkt 238001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\22OCP3.SGM 22OCP3 mstockstill on DSK4VPTVN1PROD with PROPOSALS3 64192 Federal Register / Vol. 80, No. 204 / Thursday, October 22, 2015 / Proposed Rules DEPARTMENT OF HOMELAND Collins, Program Manager, Docket II. Abbreviations SECURITY Operations, telephone 202–366–9826, DHS—Department of Homeland Security toll free 1–800–647–5527. E.O.—Executive Order Coast Guard SUPPLEMENTARY INFORMATION: FR—Federal Register IBC Code—International Code for the 46 CFR Parts 30, 150, and 153 Table of Contents for Preamble Construction and Equipment of Ships Carrying Dangerous Chemicals in Bulk [Docket No. USCG–2013–0423] I. Public Participation and Comments II. Abbreviations IMO—International Maritime Organization RIN 1625–AB94 III. Discussion MARPOL—International Convention for the IV. Regulatory Analyses Prevention of Pollution from Ships, 1973 2013 Liquid Chemical Categorization A. Regulatory Planning and Review MEPC—Marine Environment Protection Updates B. Small Entities Committee C. Assistance for Small Entities NLS—Noxious liquid substance AGENCY: Coast Guard, DHS. D. Collection of Information SOLAS—International Convention for the ACTION: Supplemental notice of E. Federalism Safety of Life at Sea proposed rulemaking. F. Unfunded Mandates Reform Act §—Section Symbol G. Taking of Private Property U.S.C.—United States Code H. Civil Justice Reform SUMMARY: The Coast Guard proposes III. Discussion additional updates and revisions to I.