40 CFR Ch. I (7–1–97 Edition) § 60.488
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

02/06/2019 12:05 PM Appendix 3745-21-09 Appendix A
ACTION: Final EXISTING DATE: 02/06/2019 12:05 PM Appendix 3745-21-09 Appendix A List of Organic Chemicals for which Paragraphs (DD) and (EE) of Rule 3745-21-09 of the Administrative Code are Applicable Organic Chemical Organic Chemical Acetal Benzaldehyde Acetaldehyde Benzamide Acetaldol Benzene Acetamide Benzenedisulfonic acid Acetanilide Benzenesulfonic acid Acetic acid Benzil Acetic Anhydride Benzilic acid Acetone Benzoic acid Acetone cyanohydrin Benzoin Acetonitrile Benzonitrile Acetophenone Benzophenone Acetyl chloride Benzotrichloride Acetylene Benzoyl chloride Acrolein Benzyl alcohol Acrylamide Benzylamine Acrylic acid Benzyl benzoate Acrylonitrile Benzyl chloride Adipic acid Benzyl dichloride Adiponitrile Biphenyl Alkyl naphthalenes Bisphenol A Allyl alcohol Bromobenzene Allyl chloride Bromonaphthalene Aminobenzoic acid Butadiene Aminoethylethanolamine 1-butene p-aminophenol n-butyl acetate Amyl acetates n-butyl acrylate Amyl alcohols n-butyl alcohol Amyl amine s-butyl alcohol Amyl chloride t-butyl alcohol Amyl mercaptans n-butylamine Amyl phenol s-butylamine Aniline t-butylamine Aniline hydrochloride p-tertbutyl benzoic acid Anisidine 1,3-butylene glycol Anisole n-butyraldehyde Anthranilic acid Butyric acid Anthraquinone Butyric anhydride Butyronitrile Caprolactam APPENDIX p(183930) pa(324943) d: (715700) ra(553210) print date: 02/06/2019 12:05 PM 3745-21-09, Appendix A 2 Carbon disulfide Cyclohexene Carbon tetrabromide Cyclohexylamine Carbon tetrachloride Cyclooctadiene Cellulose acetate Decanol Chloroacetic acid Diacetone alcohol -

Agrimer™ Polyvinylpyyrolidone (PVP)
agrimer ™ polyvinylpyyrolidone (PVP) binder, dispersant rheology, modifier, film former, complexing agent Agrimer™ polyvinylpyrrolidone (PVP) this brochure is divided into two main segments suggested applications General properties and uses 2-10 ¢ complexing agent Agricultural case studies 10 ¢ stabilizers / co-dispersants These case studies highlight the uses of Agrimer™ ¢ binders in dry / wet granulation and extrusion (dry compaction / fluidized-bed spray drying process) polymers in seed coatings, granule and tablet binders and as dispersants. ¢ film-forming agents / binders in seed coatings, dips and pour-ons general properties and uses ¢ biological stabilization ¢ water binding / anti-transpiration properties Agrimer™ PVP products are linear, non-ionic polymers that are soluble in water and many organic solvents. ¢ solubility enhancers via co-precipitation or They are pH stable, and have adhesive, cohesive thermal extrusion and binding properties. The unique ability to adsorb ¢ dye-binding agent on a host of active ingredients makes Agrimer™ PVP regulatory status homopolymers preferred co-dispersants in many The Agrimer™ PVP products listed in this brochure are formulations. Agrimer™ homopolymers have a high exempt from the requirement of a tolerance under glass transition temperature. 40 CFR 180.960. Lower molecular weight (Mw) Agrimer™ polymers (Agrimer™ 15 and Agrimer™ 30) are suitable for physical and chemical properties applications where dusting is a concern, such as The Agrimer™ polymers, a family of homopolymers of seed coatings and agglomeration. Higher Mw polyvinylpyrrolidone, are available in different viscosity Agrimer™ polymers (Agrimer™ 90 and Agrimer™ 120) can grades, ranging from very low to very high molecular build formulation viscosity faster and provide excellent weight. This range, coupled with their solubility in binding and film forming properties. -

Euthanasia of Experimental Animals
EUTHANASIA OF EXPERIMENTAL ANIMALS • *• • • • • • • *•* EUROPEAN 1COMMISSIO N This document has been prepared for use within the Commission. It does not necessarily represent the Commission's official position. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int) Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 1997 ISBN 92-827-9694-9 © European Communities, 1997 Reproduction is authorized, except for commercial purposes, provided the source is acknowledged Printed in Belgium European Commission EUTHANASIA OF EXPERIMENTAL ANIMALS Document EUTHANASIA OF EXPERIMENTAL ANIMALS Report prepared for the European Commission by Mrs Bryony Close Dr Keith Banister Dr Vera Baumans Dr Eva-Maria Bernoth Dr Niall Bromage Dr John Bunyan Professor Dr Wolff Erhardt Professor Paul Flecknell Dr Neville Gregory Professor Dr Hansjoachim Hackbarth Professor David Morton Mr Clifford Warwick EUTHANASIA OF EXPERIMENTAL ANIMALS CONTENTS Page Preface 1 Acknowledgements 2 1. Introduction 3 1.1 Objectives of euthanasia 3 1.2 Definition of terms 3 1.3 Signs of pain and distress 4 1.4 Recognition and confirmation of death 5 1.5 Personnel and training 5 1.6 Handling and restraint 6 1.7 Equipment 6 1.8 Carcass and waste disposal 6 2. General comments on methods of euthanasia 7 2.1 Acceptable methods of euthanasia 7 2.2 Methods acceptable for unconscious animals 15 2.3 Methods that are not acceptable for euthanasia 16 3. Methods of euthanasia for each species group 21 3.1 Fish 21 3.2 Amphibians 27 3.3 Reptiles 31 3.4 Birds 35 3.5 Rodents 41 3.6 Rabbits 47 3.7 Carnivores - dogs, cats, ferrets 53 3.8 Large mammals - pigs, sheep, goats, cattle, horses 57 3.9 Non-human primates 61 3.10 Other animals not commonly used for experiments 62 4. -

Chemical Compatibility Chart X
Chemical Compatibility Chart Below is a chart adapted from the CRC Laboratory Handbook, which groups various chemicals in to 23 groups with examples and incompatible chemical groups. This chart is by no means complete but it will aid in making decisions about storage. For more complete information please refer to the MSDS for the specific chemical. Examples of each group can be found on the next pages. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Monomers Polymerizable Esters Alcohols, Glycols, Glycol Ether Amines and Alkanolamines Halogenated Compounds Aldehydes Acetaldehyde Saturated Hydrocar Aromatic Hydrocarbons Acid Anhydrides Alkylene Oxides Inorganic Acids Petrolium Oils Organic Acids Cyanohydrins Phosphorus Ammonia Group Halogens Ketones Caustics Phenols Nitriles Olefins Ethers Number/Chemical Esters Type bons Inorganic 1 x x x x x x x x x x x x x x x x x Acids 2 Organic Acids x x x x x x x x x x 3 Caustics x x x x x x x x x x x x x Amines and 4 x x x x x x x x x x x x Alkanolamines Halogenated 5 x x x x x x Compounds Alcohols, 6 Glycols, Glycol x x x x x x Ether Aldehydes 7 x x x x x x x x x x x x Acetaldehyde 8 Ketones x x x x x x Saturated 9 x Hydrocarbons Aromatic 10 x x Hydrocarbons 11 Olefins x x x 12 Petrolium Oils x 13 Esters x x x x x Monomers 14 Polymerizable x x x x x x x x x x x x Esters 15 Phenols x x x x x x x Alkylene 16 x x x x x x x x x x x x Oxides 17 Cyanohydrins x x x x x x x x x 18 Nitriles x x x x x x 19 Ammonia x x x x x x x x x x x 20 Halogens x x x x x x x x x x x x x x 21 Ethers x x x 22 Phosphorus x x x x Acid 23 x x x x x x x x x x Anhydrides X - Indicates chemicals that are incompatible and should not be stored together. -
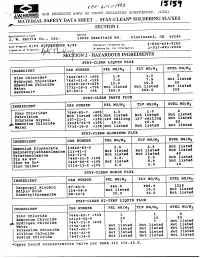
Section 2 - Hazardous Ingredients
rev- OUR PRODUCTS HAVE NO OZONE DEPLETING SUBSTANCES. (ODS) SAFETY DATA SHEET - STAY-CLEAN* SOLDERING FLUXES SECTION 1 Address Manufacturer's Name Cincinnati, OH 45242 j. w. Harris Co., Inc. 10930 Deerfield Rd. 1-800-424-9300 ES 8/92 Emergency Telephone No. Date Prepared 6/93 SUP 1-513-891-2000 Telephone Mo. for Information Signature of Preparer: Section 2 - hazardous ingredients STAY-CLEAN LIQUID FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 7.5 Not listed Hydrogen chloride* 7647-01-0 <15% 7.0 12125-02-9<25% 10.0 10.0 20 Ammonium chloride Not listed 7732-18-5 <70% Not listed Not listed Water 260.0 262.0 325 Methanol* 67-56-1 <5% STAY-CLEAN PASTE FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 Not listed Not listed Not listed Petrolatum Not listed <80% 127 ceiling Not listed Ethylene Glycol 107-21-1 <15% 125 ceiling 10.0 20 Ammonium Chloride 12125-02-9 <10% 10.0 Not listed Not listed Not listed Water 7732-18-5 <10% STAY-CLEAN ALUMINUM FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 2.5 Not listed Ammonium Fluoborate 13826-83-0 2.5 Not listed Not listed Aminoethylethanolamine 111-41-1 Not listed Not listed 60 Triethanolamine 102-71-6 Not listed 2.0 Not listed Tin as Sn* 7440-31-5 <10% 2.0. -

Förordning Om Ändring I Förordningen (1992:1554) Om Kontroll Av Narkotika;
Príloha 11 k rozhodnutiu švédskych úradov vlády 22. februára 2018 § 79 1. ------IND- 2018 0079 S-- SK- ------ 20180302 --- --- PROJET Zbierka zákonov Švédska SFS Published on issued on 1 March 2018. The government hereby lays down1 that Annex 1 to the Ordinance (1992:1554) on the control of narcotic drugs2 shall read as set out below. This ordinance shall enter into force on 10 April 2018. On behalf of the Government ANNIKA STRANDHÄLL Lars Hedengran (Ministry of Health and Social Affairs) 1 See Directive (EU) 2015/1535 of the European Parliament and of the Council of 9 September 2015 laying down a procedure for the provision of information in the field of technical regulations and of rules on Information Society services. 2 Ordinance reprinted as 1993:784. 1 SFS Annex 13 List of substances to be considered narcotic drugs according to the Narcotic Drugs Punishments Act Stimulants of the central nervous system ethylamphetamine (2-ethylamino-1-phenylpropane) fenethylline [1-phenyl-1-piperidyl-(2)-methyl]acetate 1-phenyl-2-butylamine N-hydroxyamphetamine propylhexedrine 4-methylthioamphetamine (4-MTA) modafinil 4-methoxy-N-methylamphetamine (PMMA, 4-MMA) 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) 4-iodo-2,5-dimethoxyphenethylamine (2C-I) 2,4,5-trimethoxyamphetamine (TMA-2) 4-methylmethcathinone (mephedrone) 4-fluoramphetamine 1-(4-methoxyphenyl)-2-(methylamino)propan-1-one (methedrone) 1-(1,3-benzodioxol-5-yl)-2-pyrrolidin-1-yl-pentan-1-one (MDPV) 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butan-1-one -

TERTIARY ACETYLENIC ALCOHOLS Compound, Ethchlorvynol (S 94) Is
36 H. ISBELL & T. L. CHRUSCIEL TERTIARY ACETYLENIC ALCOHOLS Only two compounds are listed (Table IV). Of 152. Csarza-Perez, J., Lal, S. & Lopez, E. (1967) Med. these, methylpentynol (S 95) is a weak, short-acting Serv. J. Can., 23, No. 5, 775-778 (Addiction to hypnotic. Sales have been low and few instances chlorvynol) of abuse have been reported.155' 157 The other 153. Essig, C. F. (1964) Clin. Pharmacol., 5, 334 (Addic- compound, ethchlorvynol (S 94) is more potent and tion to sedatives and tranquilizers) instances of abuse are more frequent. There is 154. Government of the United States of America, stiong clinical documentation for abrupt withdrawal US Food and Drug Administration (1965) Back- of ethchlorvynol being followed by convulsions and ground material supplied to Advisory Committee delirium.151-154, 156 Accordingly, ethchlorvynol must on Abuse of Stimulant and Depressant Drugs, be judged to have moderate abuse potential and 27 December 1965, Washington, D.C. 155. Hitsche, B. & Herbst, A. (1967) Psychiat. et methylpentynol must, by analogy, be regarded as Neurol. (Basel), 153, 308-318 (Beobachtungen having dependence potential equivalent to that of bei Missbrauch von Methylpentinol) ethchlorvynol. 156. Hudson, H. S. & Walker, H. I. (1961) Amer. J. Psychiat., 118, 361 (Withdrawal symptoms REFERENCES following ethchlorvynol (Placidyl) dependence) 157. Marley, E. & Bartholomew, A. A. (1958) J. Neurol. 151. Cohn, C. H. (1959) Canad. med. Ass. J., 81, 733 Neurosurg. Psychiat., 21, 129-140 (Clinical (Intoxication by ethchlorvynol-Placidyl) aspects of susceptibility of methylpentol) CYCLIC ETHERS The only compound in this group is paraldehyde dogs physically dependent on barbital. -

Investigations in Fish Control
INVESTIGATIONS IN FISH CONTROL 29. Efficacy of Methylpentynol as an Anesthetic on Four Salmonids 30. Toxicity of Methylpentynol to Selected Fishes 31. Annotated Bibliography on Methylpentynol United States Department of the Interior Fish and Wildlife Service Bureau of Sport Fisheries and Wildlife INVESTIGATIONS IN FISH CONTROL Investigations in Fish Control, published by the Bureau of Sport Fisheries and Wildlife, in clude reports on the results of work at the Bureau's Fish Control Laboratories at La Crosse, Wis., and Warm Springs, Ga., and reports of other studies related to that work. Though each report is regarded as a separate publication, several may be issued under a single cover, for economy. Current reports in this series are (Reports 1 and 2 are in one cover.) 1. Laboratories and Methods for Screening Fish-Control Chemicals, by Robert E. Lennon and Charles R. Walker. 1964. 15 p. 2. Preliminary Observations on the Toxicity of Antimycin A to Fish and Other Aquatic Animals, by Charles R. Walker, Robert E. Lennon, and Bernard L. Berger. 1964. 18 p. (Reports 3 through 8 are in one cover.) 3. Minimum Lethal Levels of Toxaphene as a Piscicide in North Dakota Lakes, by Dale L. Henegar. 1966. 16 p. 4. Effects of Toxaphene on Plankton and Aquatic Invertebrates in North Dakota Lakes, by Robert G. Needham. 1966. 16 p. 5. Growth Rates of Yellow Perch in Two North Dakota Lakes After Population Reduction with Toxaphene, by Donald C. Warnick. 1966. 9 p. 6. Mortality of Some Species of Fish to Toxaphene at Three Temperatures, by Mahmoud Ahmed Mahdi. -

BEMEGRIDE ANALEPSIS the Administration of Convulsant Doses of Bemegride (60 Mg./Kg
APRIL 12, 1958 TUBERCULIN SENSITIVITY IN KUWAITI SCHOOLS BDI&nm 871 BIBLIOaRAPHY structurally unrelated hypnotics. Bemegride is generally a Abboud. M. A., and Abdin, Z. H. (1954). Gaz. Egypt. paediat. Ass., 2, 71. more potent analeptic to this latter class of substance. Bicknell. F., and Prescott, F. (1946). The Vitamins in Medicine, 2nd ed. We wish to emphasize the high margin of safety asso- Heinemann, London. Clarke, B. R. (1952). Causes and Prevention of Tuberculosis. Livingstone, ciated with the administration of bemegride in the relief of Edinburgh. moderate hypnosis induced by both classes of drugs. Faber, K. (1938). Acta tubere. scand., 12, 287. Hart, P. D'Arcy (1932). Spec. Rep. Scr. med. Res. Coun. (Lond.), No. 164. Significant reduction in sleeping-time (p .0.001) has been Preventive Medicine Dept. (1957). Reports of Antituberculosis Section. obtained by the administration of mildly convulsant doses P.H.D., Kuwait. -(1957). Reports of School Health Section. P.H.D., Kuwait. of bemegride (20 mg./kg. each 15 minutes) to mice narco- Yelton, S. E. (1946). Publ. Hlth Rep. (Wash.), 61, 1144. tized with hypnotics of 15 structurally different types. A few transient and minimal signs of toxicity have been occa- sionally observed in the case of only two drugs (urethane and 8-methyl-,8-n-amylglutarimide). BEMEGRIDE ANALEPSIS The administration of convulsant doses of bemegride (60 mg./kg. stat. and up to 6 doses of 30 mg./kg. at seven- BY minute intervals) to mice deeply narcotized with the same series of structurally diverse hypnotics also resulted in a A. SHULMAN, M.B., B.Sc. -

Chemical Compatibility Chart
Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride Oleum Methylene chloride Phosphoric acid Monochlorodifluoromethane Sulfuric acid Perchloroethylene Propylene dichloride Group 2: Organic Acids 1,2,4-Trichlorobenzene Acetic acid 1,1,1-Trichloroethane Butyric acid (n-) Trichloroethylene Formic acid Trichlorofluoromethane Propionic acid Rosin Oil Group 6: Alcohols, Glycols and Glycol Ethers Tall oil Allyl alcohol Amyl alcohol Group 3: Caustics 1,4-Butanediol Caustic potash solution Butyl alcohol (iso, n, sec, tert) Caustic soda solution Butylene -

Laws and Regulations Promulgated to Give Effect to the Provisions of the International Treaties on Narcotic Drugs and Psychotropic Substances
UNITED NATIONS E/NL 1986/1-4 30 September 1986 ENGLISH ONLY * LAWS AND REGULATIONS PROMULGATED TO GIVE EFFECT TO THE PROVISIONS OF THE INTERNATIONAL TREATIES ON NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES In accordance with the relevant articles of the international treaties on narcotic drugs and psychotropic substances, the Secretary-General has the honour to communicate the following legislative texts. SWEDEN Communicated by the Government of Sweden NOTE BY THE SECRETARIAT (a) Some editing of texts may be done by the Secretariat in the interest of clarity. In this connection, words in square brackets [ ] have been added or changed by the Secretariat. (b) Only passages directly relevant to the control of narcotic drugs or psycho• tropic substances have been reproduced in this document. Non-relevant parts of laws and regulations have been deleted by the Secretariat; such deletions are indicated by [...]. INDEX PaSe E/NL.1986/1 Law Amending the Law (1960:*H8) on Penalties for the Smuggling of Goods E/NL.1986/2 Act Amending the Penal Law on Narcotics (1968:6U) 3 E/NL.1986/3 Ordinance Amending the Ordinance (1983:366) Classifying k Certain Substances as Narcotic Drugs E/NL.1986A National Board of Health and Welfare. Notification on List of Narcotics, 2 May 1985 SOSFS 1985:9 Note by the Secretariat : The present document is a direct reproduction of the texts received by the Secretariat. V.86-60352 K/NL.1986/1-4 page 2 E/NL.1986/1 SFS 1985:10 Issued by the printer's on 22 January 1985 Law amending the Law (1960:418) on Penalties for the Smuggling of Goods promulgated on 10 January 1985. -

Fr9de98b.Pdf
Federal Register / Vol. 63, No. 236 / Wednesday, December 9, 1998 / Proposed Rules 68037 (ii) The required mass removal is downstream of the point of Ci=Concentration of VOC at the point of calculated by summing the required determination with adjustment for determination, parts per million by mass removal for all wastewater streams concentration change made according to weight. combined for treatment when § 60.782(b)(6) of this subpart. Fri=Fraction removal value of a VOC. complying with § 60.779(g)(1)(i) or (g)(2) Concentration measurements to Follow the procedures in § 60.778 of this subpart. determine AMR shall be taken at the of this subpart to develop a stream- (5) The AMR calculation procedure inlet and outlet to the treatment process specific list of VOC. Follow the for non-combustion treatment processes and as provided in paragraph (a)(7) of procedures in appendix J of this including closed biological treatment this section for a series of treatment part to determine Fr values. processes. The AMR shall be calculated processes. Wastewater samples shall be 10 9=Conversion factor, mg/kg * l/m 3. as follows: collected using sampling procedures (4) The required mass removal is which minimize loss of organic calculated by adding together the = − AMR() QMWa QMW b () Eqn WW10 compounds during sample collection required mass removal for each Group 1 Where: and analysis and maintain sample wastewater stream to be combined for AMR=Actual mass removal of VOC integrity per § 60.782(b)(5)(ii) of this treatment. achieved by treatment process or subpart. The method shall be an (5) Actual mass removal calculation series of treatment processes, analytical method for wastewater which procedure for open or closed aerobic kilograms per hour.