Department of Homeland Security
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography
foods Article Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography Cátia Martins 1 , Tiago Brandão 2, Adelaide Almeida 3 and Sílvia M. Rocha 1,* 1 Departamento de Química & LAQV-REQUIMTE, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] 2 Super Bock Group, Rua do Mosteiro, 4465-703 Leça do Balio, Portugal; [email protected] 3 Departamento de Biologia & CESAM, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-234-401-524 Received: 30 July 2020; Accepted: 6 September 2020; Published: 11 September 2020 Abstract: Foodomics, emergent field of metabolomics, has been applied to study food system processes, and it may be useful to understand sensorial food properties, among others, through foods metabolites profiling. Thus, as beer volatile components represent the major contributors for beer overall and peculiar aroma properties, this work intends to perform an in-depth profiling of lager beer volatile metabolites and to generate new data that may contribute for molecules’ identification, by using multidimensional gas chromatography. A set of lager beers were used as case-study, and 329 volatile metabolites were determined, distributed over 8 chemical families: acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. From these, 96 compounds are reported for the first time in the lager beer volatile composition. Around half of them were common to all beers under study. Clustering analysis allowed a beer typing according to production system: macro- and microbrewer beers. Monoterpenic and sesquiterpenic compounds were the chemical families that showed wide range of chemical structures, which may contribute for the samples’ peculiar aroma characteristics. -

Agrimer™ Polyvinylpyyrolidone (PVP)
agrimer ™ polyvinylpyyrolidone (PVP) binder, dispersant rheology, modifier, film former, complexing agent Agrimer™ polyvinylpyrrolidone (PVP) this brochure is divided into two main segments suggested applications General properties and uses 2-10 ¢ complexing agent Agricultural case studies 10 ¢ stabilizers / co-dispersants These case studies highlight the uses of Agrimer™ ¢ binders in dry / wet granulation and extrusion (dry compaction / fluidized-bed spray drying process) polymers in seed coatings, granule and tablet binders and as dispersants. ¢ film-forming agents / binders in seed coatings, dips and pour-ons general properties and uses ¢ biological stabilization ¢ water binding / anti-transpiration properties Agrimer™ PVP products are linear, non-ionic polymers that are soluble in water and many organic solvents. ¢ solubility enhancers via co-precipitation or They are pH stable, and have adhesive, cohesive thermal extrusion and binding properties. The unique ability to adsorb ¢ dye-binding agent on a host of active ingredients makes Agrimer™ PVP regulatory status homopolymers preferred co-dispersants in many The Agrimer™ PVP products listed in this brochure are formulations. Agrimer™ homopolymers have a high exempt from the requirement of a tolerance under glass transition temperature. 40 CFR 180.960. Lower molecular weight (Mw) Agrimer™ polymers (Agrimer™ 15 and Agrimer™ 30) are suitable for physical and chemical properties applications where dusting is a concern, such as The Agrimer™ polymers, a family of homopolymers of seed coatings and agglomeration. Higher Mw polyvinylpyrrolidone, are available in different viscosity Agrimer™ polymers (Agrimer™ 90 and Agrimer™ 120) can grades, ranging from very low to very high molecular build formulation viscosity faster and provide excellent weight. This range, coupled with their solubility in binding and film forming properties. -

779 Part 770—Interpretations
Pt. 770 15 CFR Ch. VII (1–1–21 Edition) in the item that qualitatively affect the per- roller bearings and parts). This applies formance of the U.S. and foreign items; to separate shipments of anti-friction (vi) Evidence of the interchangeability of bearings or bearing systems and anti- U.S. and foreign items; friction bearings or bearing systems (vii) Patent descriptions for the U.S. and foreign items; shipped with machinery or equipment (viii) Evidence that the U.S. and foreign for which they are intended to be used items meet a published industry, national, or as spares or replacement parts. international standard; (2) An anti-friction bearing or bear- (ix) A report or eyewitness account, by ing system physically incorporated in a deposition or otherwise, of the foreign item’s segment of a machine or in a complete operation; machine prior to shipment loses its (x) Evidence concerning the foreign manu- identity as a bearing. In this scenario, facturers’ corporate reputation; (xi) Comparison of the U.S. and foreign end the machine or segment of machinery item(s) made from a specific commodity, containing the bearing is the item sub- tool(s), device(s), or technical data; or ject to export control requirements. (xii) Evidence of the reputation of the for- (3) An anti-friction bearing or bear- eign item including, if possible, information ing system not incorporated in a seg- on maintenance, repair, performance, and ment of a machine prior to shipment, other pertinent factors. but shipped as a component of a com- plete unassembled (knocked-down) ma- SUPPLEMENT NO. -

Volatile Organic Compounds from Books
1 Measuring the emission of volatile organic compounds from books Velson Horie Research Project Manager The British Library What is happening to our books? presevation of folding endurance DP X. Zou, T. Uesaka, N. Gurnagul, Prediction of paper permanence by accelerated aging. Part I: Kinetic analysis of the 3 aging process , Cellulose, 1996, 3, 243-267. A Few Statistics •Formal beginning in 1753 as the library of The British Museum •The British Library formed in 1973 from many collections •New St Pancras building opened in 1998 •150m collection items on 640km of shelves, •£131m budget, 1900 staff 4 Additional Storage Programme - Boston Spa •7 million collection items •263 km, 12,000 tonne of stock •Reduced oxygen (16%) •Robotic book handling •What are the long term effects? 5 Preserving Newspapers •33 km of stock •5,300 tonne of stock •1.4 tonne/y VOC production •3,800 years till all evaporated 6 Major UK libraries and archives Cambridge University Library (CUL) 7m printed items The British Library (BL) 150m items National Library of Scotland (NLS) 14m items National Library of Wales (NLW) 6m printed items Oxford University Library(OULS) 11m items Trinity College Dublin Library (TCD) 4m printed items The National Archives (TNA) National Archives of Scotland (NAS) 7 Condition assessment Preservation Assessment Survey Strength Colour pH Molecular weight Furnish SurveNIR VOCs 8 The “real thing” is important to people E-books sales have been slow to take off. CafeScribe is sending every e-textbook purchaser a scratch and sniff sticker with a musty “old book” smell. By placing these stickers on their computers, they can give their e-books the same musty book smell they know and love from used textbooks. -

Chemical Compatibility Chart X
Chemical Compatibility Chart Below is a chart adapted from the CRC Laboratory Handbook, which groups various chemicals in to 23 groups with examples and incompatible chemical groups. This chart is by no means complete but it will aid in making decisions about storage. For more complete information please refer to the MSDS for the specific chemical. Examples of each group can be found on the next pages. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Monomers Polymerizable Esters Alcohols, Glycols, Glycol Ether Amines and Alkanolamines Halogenated Compounds Aldehydes Acetaldehyde Saturated Hydrocar Aromatic Hydrocarbons Acid Anhydrides Alkylene Oxides Inorganic Acids Petrolium Oils Organic Acids Cyanohydrins Phosphorus Ammonia Group Halogens Ketones Caustics Phenols Nitriles Olefins Ethers Number/Chemical Esters Type bons Inorganic 1 x x x x x x x x x x x x x x x x x Acids 2 Organic Acids x x x x x x x x x x 3 Caustics x x x x x x x x x x x x x Amines and 4 x x x x x x x x x x x x Alkanolamines Halogenated 5 x x x x x x Compounds Alcohols, 6 Glycols, Glycol x x x x x x Ether Aldehydes 7 x x x x x x x x x x x x Acetaldehyde 8 Ketones x x x x x x Saturated 9 x Hydrocarbons Aromatic 10 x x Hydrocarbons 11 Olefins x x x 12 Petrolium Oils x 13 Esters x x x x x Monomers 14 Polymerizable x x x x x x x x x x x x Esters 15 Phenols x x x x x x x Alkylene 16 x x x x x x x x x x x x Oxides 17 Cyanohydrins x x x x x x x x x 18 Nitriles x x x x x x 19 Ammonia x x x x x x x x x x x 20 Halogens x x x x x x x x x x x x x x 21 Ethers x x x 22 Phosphorus x x x x Acid 23 x x x x x x x x x x Anhydrides X - Indicates chemicals that are incompatible and should not be stored together. -
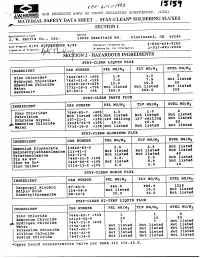
Section 2 - Hazardous Ingredients
rev- OUR PRODUCTS HAVE NO OZONE DEPLETING SUBSTANCES. (ODS) SAFETY DATA SHEET - STAY-CLEAN* SOLDERING FLUXES SECTION 1 Address Manufacturer's Name Cincinnati, OH 45242 j. w. Harris Co., Inc. 10930 Deerfield Rd. 1-800-424-9300 ES 8/92 Emergency Telephone No. Date Prepared 6/93 SUP 1-513-891-2000 Telephone Mo. for Information Signature of Preparer: Section 2 - hazardous ingredients STAY-CLEAN LIQUID FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 7.5 Not listed Hydrogen chloride* 7647-01-0 <15% 7.0 12125-02-9<25% 10.0 10.0 20 Ammonium chloride Not listed 7732-18-5 <70% Not listed Not listed Water 260.0 262.0 325 Methanol* 67-56-1 <5% STAY-CLEAN PASTE FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 1.0 Zinc Chloride* 7646-85-7 <40% 1.0 Not listed Not listed Not listed Petrolatum Not listed <80% 127 ceiling Not listed Ethylene Glycol 107-21-1 <15% 125 ceiling 10.0 20 Ammonium Chloride 12125-02-9 <10% 10.0 Not listed Not listed Not listed Water 7732-18-5 <10% STAY-CLEAN ALUMINUM FLUX PEL MG/M3 TLV MG/M3 STEL MG/M3 INGREDIENT CAS NUMBER 2.5 Not listed Ammonium Fluoborate 13826-83-0 2.5 Not listed Not listed Aminoethylethanolamine 111-41-1 Not listed Not listed 60 Triethanolamine 102-71-6 Not listed 2.0 Not listed Tin as Sn* 7440-31-5 <10% 2.0. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth Et Al
US 2011 0028732A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth et al. (43) Pub. Date: Feb. 3, 2011 (54) NITRATED HYDROCARBONS, (86). PCT No.: PCT/US2009/0399.01 DERIVATIVES, AND PROCESSES FOR THEIR MANUFACTURE S371 (c)(1), (2), (4) Date: Sep. 27, 2010 (75) Inventors: Daniel M. Trauth, Crystal Lake, IL Related U.S. Application Data (US); George D. Green, Cary, IL (US); Raymond J. Swedo, Mt. (60) Eyal application No. 61/045.380, filed on Apr. Prospect, IL (US); Richard L. s James, Eros, LA (US); Ian A. Publication Classification Tomlinson, Midland, MI (US) (51) Int. Cl. C07D 263/04 (2006.01) Correspondence Address: C07C 205/05 (2006.01) The Dow Chemical Company C07C 205/01 (2006.01) P.O. BOX 1967, 2040 Dow Center CD7C 205/06 (2006.01) Midland, MI 48641 (US) C07C 215/02 (2006.01) C07C 239/08 (2006.01) (73) Assignees: ANGUS CHEMICAL (52) U.S. Cl. ......... 548/215; 7.3,So COMPANY, Buffalo Grove, IL s s (US): GLOBAL (57) ABSTRACT TECHNOLOGIES INC. , MidlandM1Clland, Provided is a process for the formation of nitrated compounds MI (US) by the nitration of hydrocarbon compounds with- dilute- - nitric acid. Also provided are processes for preparing industrially (21) Appl. No.: 12/934,817 useful downstream derivatives of the nitrated compounds, as well as novel nitrated compounds and derivatives, and meth (22) PCT Filed: Apr. 8, 2009 ods of using the derivatives in various applications. US 2011/0028732 A1 Feb. 3, 2011 NITRATED HYDROCARBONS, 0009. The invention further provides methods of using the DERIVATIVES, AND PROCESSES FOR THEIR nitrated hydrocarbons and derivatives thereof in various MANUFACTURE applications. -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Cambridge CB2 3EG (Received 14 June 1990)
Journal of Physiology (1991), 437, pp. 431-448 431 With 11 figures Printed in Great Britain ACTIONS OF n-ALCOHOLS ON NICOTINIC ACETYLCHOLINE RECEPTOR CHANNELS IN CULTURED RAT MYOTUBES BY R. D. MURRELL*, M. S. BRAUNt AND THE LATE D. A. HAYDON From the Physiological Laboratory, University of Cambridge, Downing Street, Cambridge CB2 3EG (Received 14 June 1990) SUMMARY 1. The actions of the n-alcohols from pentanol to dodecanol on nicotinic acetylcholine receptor (nAChR) channels were investigated by recording single ACh- activated channel activity from inside-out membrane patches isolated from cultured rat myotubes. Alcohols were applied to the cytoplasmic side of the membrane; aqueous concentrations ranged from 11 7 mM-pentanol to 0-02 mm-dodecanol. 2. The intermediate-chain alcohols (pentanol to octanol) caused channel currents to fluctuate between the fully open and closed state level so that openings occurred in bursts interrupted by brief gaps. Closed time distributions were fitted well with two exponential components, the fast component representing the closures within a burst. The number of gaps within a burst was dependent on alcohol concentration whereas gap duration was independent of concentration but increased with increasing chain length of the alcohol up to octanol. 3. Nonanol and decanol reduced the mean duration of bursts of openings but did not cause an increase in the number of short closed intervals within a burst. Beyond decanol there was a decline in the ability of the n-alcohols to affect channel function. A saturated solution of undecanol (0-07 mM) reduced the mean open time by 33+17 %, whereas a saturated solution of dodecanol had no significant effect. -

KUNDU-THESIS.Pdf
Acknowledgement I would like to thank my advisor Prof. Zachary T. Ball for teaching me everything I know in synthetic skills during my first year and guiding me through the rest of my time here at Rice. He has helped me better myself through constant constructive criticism, both in research and writing. I would also like to thank past and present Ball group members, Dr. Brian Popp, Dr. Alex Zaykov, Dr. Jessica Herron, Vincenzo Russo, Ramya Sambasivan, Cara Bovet, Zhen Chen, Dr. Jane Coughlin, Farrukh Vohidov, Matt Minus, Rob Ferguson, Julian Cooper. They have been a very good support in discussing research, exchanging ideas, and have become great pals in the last few years. Especially, Brian and Ramya’s encouragement in tough times is really valued. I thank my friends Rajkishore Barik and Meenu Adhikari for making Houston a second home. Again, Ramya Sambasivan for being a very good friend and always a willing ear to vent out frustrations associated with the graduate life and Avani Verma for bringing all kinds of fun in life and being so encouraging during my panic moments in thesis writing process. I would like to take this opportunity to express my gratitude and respect for my father Kuru Ram, and mother Amita Kundu, for the confidence and faith they always had in me, my sister Aruna Hajra who always supported me in my endeavors. I would like to thank my fiancée Soumya Sarkar, for his never wavering trust, love and support throughout my Ph.D in our 6 yr long, long- distance relation. Last but not least thanks to everyone who cared! -Rituparna Kundu Abstract Developing Dirhodium-Complexes for Protein Inhibition and Modification & Copper-Catalyzed Remote Chlorination of Alkyl-Hydroperoxides by Rituparna Kundu The work describes the development of a new class of protein-inhibitors for protein-protein interactions, based on metallopeptides comprised of a dirhodium metal center. -

Chemical Compatibility Chart
Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride Oleum Methylene chloride Phosphoric acid Monochlorodifluoromethane Sulfuric acid Perchloroethylene Propylene dichloride Group 2: Organic Acids 1,2,4-Trichlorobenzene Acetic acid 1,1,1-Trichloroethane Butyric acid (n-) Trichloroethylene Formic acid Trichlorofluoromethane Propionic acid Rosin Oil Group 6: Alcohols, Glycols and Glycol Ethers Tall oil Allyl alcohol Amyl alcohol Group 3: Caustics 1,4-Butanediol Caustic potash solution Butyl alcohol (iso, n, sec, tert) Caustic soda solution Butylene -
![United States Patent (19) [11] 3,772,046 Knapp Et Al](https://docslib.b-cdn.net/cover/9356/united-states-patent-19-11-3-772-046-knapp-et-al-2789356.webp)
United States Patent (19) [11] 3,772,046 Knapp Et Al
United States Patent (19) [11] 3,772,046 Knapp et al. (45) Nov. 13, 1973 (54) TIO, SLURRIES HAVING A HIGH SOLIDS 3,674,528 7|1972 Bronson.............................. 106.1300 CONTENT 3,510,334 5/1970 Goodspeed.............. ... 106/300 3,506,466 4/1970 Bramekamp et al..... ... 106/300 75 Inventors: Donald Edward Knapp, Savannah, 2,933,408 4/1960 Dempster et al..... ... 106/300 Ga.; Lindo Paul Nageroni, Bound 3,702,773 1 1/1972 Hall et al............................ 1061300 Brook, N.J. (73) Assignee: American Chemical Company, Primary Examiner-Delbert E. Gantz Stamford, Conn. Assistant Examiner-James W. Hellwege 22 Filed: Jan. 17, 1972 Attorney-Roland A. Dexter (21) Appl. No.: 218,453 Related U.S. Application Data 57 ABSTRACT 63 Continuation-in-part of Ser. No. 55,286, July 15, Pigment slurries of 70% by weight solids contents are 1970, abandoned. shown to have long term stability even when exposed to typical paper making chemicals such as calcium (52) U.S. Cl................................................. 106.1300 carbonate. The aqueous slurries are formulated with (51 Int. Cl............................................... C09c 1/62 pigment containing a small but effective admixed 58) Field of Search..................................... 106/300 amount of polyhydric alcohol and an alkanolamine, and with water which contains a small but effective 56) References Cited amount of a second alkanol-amine which provides a UNITED STATES PATENTS slurry pH of at least 9. 3,649,323 3/1972 Roe et al............................ 106/300 5 Claims, No Drawings 3,772,046 2 TIO, SLURRIES HAVING A HIGH SOLIDS bonate which is frequently encountered in the produc CONTENT tion of paper.