Causes of Cyclicity of Epirrita Autumnata (Lepidoptera, Geometridae): Grandiose Theory and Tedious Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

Action Plan Pasvik-Inari Trilateral Park 2019-2028
Action plan Pasvik-Inari Trilateral Park 2019-2028 2019 Action plan Pasvik-Inari Trilateral Park 2019-2028 Date: 31.1.2019 Authors: Kalske, T., Tervo, R., Kollstrøm, R., Polikarpova, N. and Trusova, M. Cover photo: Young generation of birders and environmentalists looking into the future (Pasvik Zapovednik, О. Кrotova) The Trilateral Advisory Board: FIN Metsähallitus, Parks & Wildlife Finland Centre for Economic Development, Transport and the Environments in Lapland (Lapland ELY-centre) Inari Municipality NOR Office of the Finnmark County Governor Øvre Pasvik National Park Board Sør-Varanger Municipality RUS Pasvik Zapovednik Pechenga District Municipality Nikel Local Municipality Ministry of Natural Resource and Ecology of the Murmansk region Ministry of Economic Development of the Murmansk region, Tourism division Observers: WWF Barents Office Russia, NIBIO Svanhovd Norway Contacts: FINLAND NORWAY Metsähallitus, Parks & Wildlife Finland Troms and Finnmark County Governor Ivalo Customer Service Tel. +47 789 50 300 Tel. +358 205 64 7701 [email protected] [email protected] Northern Lapland Nature Centre Siida RUSSIA Tel. +358 205 64 7740 Pasvik State Nature Reserve [email protected] (Pasvik Zapovednik) Tel./fax: +7 815 54 5 07 00 [email protected] (Nikel) [email protected] (Rajakoski) 2 Action Plan Pasvik-Inari Trilateral Park 2019-2028 3 Preface In this 10-year Action Plan for the Pasvik-Inari Trilateral Park, we present the background of the long-lasting nature protection and management cooperation, our mutual vision and mission, as well as the concrete development ideas of the cooperation for the next decade. The plan is considered as an advisory plan focusing on common long-term guidance and cooperation. -

CURRICULUM VITAE Matthew P. Ayres
CURRICULUM VITAE Matthew P. Ayres Department of Biological Sciences, Dartmouth College, Hanover, NH 03755 (603) 646-2788, [email protected], http://www.dartmouth.edu/~mpayres APPOINTMENTS Professor of Biological Sciences, Dartmouth College, 2008 - Associate Director, Institute of Arctic Studies, Dartmouth College, 2014 - Associate Professor of Biological Sciences, Dartmouth College, 2000-2008 Assistant Professor of Biological Sciences, Dartmouth College, 1993 to 2000 Research Entomologist, USDA Forest Service, Research Entomologist, 1993 EDUCATION 1991 Ph.D. Entomology, Michigan State University 1986 Fulbright Fellowship, University of Turku, Finland 1985 M.S. Biology, University of Alaska Fairbanks 1983 B.S. Biology, University of Alaska Fairbanks PROFESSIONAL AFFILIATIONS Ecological Society of America Entomological Society of America PROFESSIONAL SERVICES Member, Board of Editors: Ecological Applications; Member, Editorial Board, Population Ecology Referee: (10-15 manuscripts / year) American Naturalist, Annales Zoologici Fennici, Bioscience, Canadian Entomologist, Canadian Journal of Botany, Canadian Journal of Forest Research, Climatic Change, Ecography, Ecology, Ecology Letters, Ecological Entomology, Ecological Modeling, Ecoscience, Environmental Entomology, Environmental & Experimental Botany, European Journal of Entomology, Field Crops Research, Forest Science, Functional Ecology, Global Change Biology, Journal of Applied Ecology, Journal of Biogeography, Journal of Geophysical Research - Biogeosciences, Journal of Economic -

What Causes Population Cycles of Forest Lepidoptera?
PERSPECTIVES to track numerical changes in their preyg. During the writing of this article, Werner What causes population cycles of forest Baltensweiler (Swiss Federal Institute of Technology, retired) sent me data on the Lepidoptera? two dominant parasitoids attacking the larch budmoth. Theoretical model-fitting (Box 1) indicates that the parasite-bud- Alan A. Berryman percapita rate of increase, R = ln(N,/N,-,), worm interaction only explains 28% of the and population density in the previous observed per-capita growth rate of the year, In N,-z, from which the effect of first- budworm and 50% of the parasitoids, Hypotheses for the causes of order correlation between R and In N,-, has which is hardly a dominant effect. regular cycles in populations of been removed. Lepidoptera with cyclical There seems to be little doubt, how- forest Lepidoptera have invoked dynamics are dominated by second-order ever, that population cycles of some for- pathogen-insect or foliage-insect feedback (PC2 > Kl) (see Fig. 1) while est Lepidoptera are the result of interac- interactions. However, the available those with more stable dynamics are domi- tions with insect parasitoids. For example, data suggest that forest caterpillar nated by first-order feedback (PC2 <Xl). Morris6 presented 11 years of data on the cycles are more likely to be the result The search for a general explanation density of blackheaded budworm (Acleris of interactions with insect parasitoids, for population cycles in forest Lepidop- UQriQnQ) caterpillars and their larval para- an old argument that seems to have tera has been approached from two major sitoids and concluded that the dynamics been neglected in recent years. -
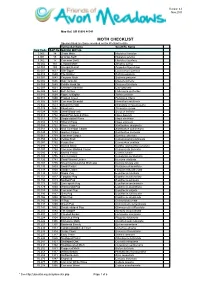
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Chemistry& Metabolism Chemical Information National Library Of
Chemistry& Metabolism Chemical Information National Library of Medicine Chemical Resources of the Environmental Health & Toxicology Information Program chemistry.org: American Chemical Society - ACS HomePage Identified Compounds — Metabolomics Fiehn Lab AOCS > Publications > Online Catalog > Modern Methods for Lipid Analysis by Liquid Chromatography/Mass Spectrometry and Related Techniques (Lipase Database) Lipid Library Lipid Library Grom Analytik + HPLC GmbH: Homepage Fluorescence-based phospholipase assays—Table 17,3 | Life Technologies Phosphatidylcholine | PerkinElmer MetaCyc Encyclopedia of Metabolic Pathways MapMan Max Planck Institute of Molecular Plant Physiology MS analysis MetFrag Scripps Center For Metabolomics and Mass Spectrometry - XCMS MetaboAnalyst Lipid Analysis with GC-MS, LC-MS, FT-MS — Metabolomics Fiehn Lab MetLIn LOX and P450 inhibitors Lipoxygenase inhibitor BIOMOL International, LP - Lipoxygenase Inhibitors Lipoxygenase structure Lypoxygenases Lipoxygenase structure Plant databases (see also below) PlantsDB SuperSAGE & SAGE Serial Analysis of Gene Expression: Information from Answers.com Oncology: The Sidney Kimmel Comprehensive Cancer Center EMBL Heidelberg - The European Molecular Biology Laboratory EMBL - SAGE for beginners Human Genetics at Johns Hopkins - Kinzler, K Serial Analysis of Gene Expression The Science Creative Quarterly » PAINLESS GENE EXPRESSION PROFILING: SAGE (SERIAL ANALYSIS OF GENE EXPRESSION) IDEG6 software home page (Analysis of gene expression) GenXPro :: GENome-wide eXpression PROfiling -

ALS Guide to Moth Trapping
ALS Beginners Guide to Moth Trapping 2nd Edition © Anglian Lepidopterist Supplies 2004 Contents 1. Trapping in the Garden 2. Moving Further Afield 3. Types of Moth Trap 4. Building Your Own Trap 5. Pests and Non-moth Species 6. Sugar and Wine Ropes 7. Larvae and Non-light Based Surveys 8. Killing and Indentification via Genitalia 9. Setting 10. Specimen Repairs 11. Mothing and the Weather 1. Trapping in your Garden The study of moths through the use of non-lethal light traps is a fascinating and rapidly growing hobby. Putting a moth trap out in the garden at dusk, and going through the catch in the morning is an easy and enjoyable way to study the range of insects visiting your garden. We are all used to the ten or so butterflies that visit most gardens, but the diversity of other lepidoptera which is using your garden may come as a surprise. Even urban gardens should attract well over 100 species during the year, with more favoured gardens easily achieving lists of 200-300 species per year. The peak months are July and August when nightly catches of several hundred moths can be expected, and for the beginner the range of species is at first site daunting. However, an hour or two with a copy of Skinner will usually sort out the majority, and having seen the species once things can only get easier! In order to maximise the variety of moths occurring in your garden a variety of strategies can be adopted. One key to success is to establish as wide a range of (native) plants as possible. -

Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Impacts of Native and Non-native plants on Urban Insect Communities: Are Native Plants Better than Non-natives? by Carl Scott Clem A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama December 12, 2015 Key Words: native plants, non-native plants, caterpillars, natural enemies, associational interactions, congeneric plants Copyright 2015 by Carl Scott Clem Approved by David Held, Chair, Associate Professor: Department of Entomology and Plant Pathology Charles Ray, Research Fellow: Department of Entomology and Plant Pathology Debbie Folkerts, Assistant Professor: Department of Biological Sciences Robert Boyd, Professor: Department of Biological Sciences Abstract With continued suburban expansion in the southeastern United States, it is increasingly important to understand urbanization and its impacts on sustainability and natural ecosystems. Expansion of suburbia is often coupled with replacement of native plants by alien ornamental plants such as crepe myrtle, Bradford pear, and Japanese maple. Two projects were conducted for this thesis. The purpose of the first project (Chapter 2) was to conduct an analysis of existing larval Lepidoptera and Symphyta hostplant records in the southeastern United States, comparing their species richness on common native and alien woody plants. We found that, in most cases, native plants support more species of eruciform larvae compared to aliens. Alien congener plant species (those in the same genus as native species) supported more species of larvae than alien, non-congeners. Most of the larvae that feed on alien plants are generalist species. However, most of the specialist species feeding on alien plants use congeners of native plants, providing evidence of a spillover, or false spillover, effect. -

Epirrita Autumnata) in Northernmost Fennoscandia
Remote Sensing of Environment 114 (2010) 637–646 Contents lists available at ScienceDirect Remote Sensing of Environment journal homepage: www.elsevier.com/locate/rse Landsat TM/ETM+ and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia Flurin Babst a,b,⁎, Jan Esper a,c, Eberhard Parlow b a Dendro Sciences Unit, Swiss Federal Research Institute WSL, Switzerland b Institute for Meteorology, Climatology and Remote Sensing, University of Basel, Switzerland c Institute of Geography, University of Mainz, Germany article info abstract Article history: We used fine-spatial resolution remotely sensed data combined with tree-ring parameters in order to assess Received 26 July 2009 and reconstruct disturbances in mountain birch (Betula pubescens) forests caused by Epirrita autumnata Received in revised form 14 October 2009 (autumnal moth). Research was conducted in the area of Lake Torneträsk in northern Sweden where we Accepted 15 November 2009 utilized five proxy parameters to detect insect outbreak events over the 19th and 20th centuries. Digital change detection was applied on three pairs of multi-temporal NDVI images from Landsat TM/ETM+ to Keywords: detect significant reductions in the photosynthetic activity of forested areas during disturbed growing Digital change detection seasons. An image segmentation gap-fill procedure was developed in order to compensate missing scan lines Dendroecology “ ” Forest disturbances in Landsat ETM+ SLC-off images. To account for a potential dependence of local outbreak levels on Insect outbreaks elevation, a digital elevation model was included in the defoliation recognition process. The resulting Defoliation damage distribution map allowed for the assessment of outbreak intensity and distribution at the stand level Betula pubescens and was combined with tree-ring data and historical documents to produce a multi-evidence outbreak detection. -

Moths of the Kingston Study Area
Moths of the Kingston Study Area Last updated 30 July 2015 by Mike Burrell This checklist contains the 783 species known to have occurred within the Kingston Study. Major data sources include KFN bioblitzes, an earlier version created by Gary Ure (2013) and the Queen’s University Biological Station list by Kit Muma (2008). For information about contributing your sightings or to download the latest version of this checklist, please visit: http://kingstonfieldnaturalists.org/moths/moths.html Contents Superfamily: Tineoidea .................................................................................................................................................... 5 Family: Tineidae ........................................................................................................................................................... 5 Subfamily: Tineinae .................................................................................................................................................. 5 Family: Psychidae ......................................................................................................................................................... 5 Subfamily: Psychinae ................................................................................................................................................ 5 Superfamily: Gracillarioidea ............................................................................................................................................. 5 Family: Gracillariidae ................................................................................................................................................... -

Biodiversity in Finnish Wilderness Areas: Historical and Cultural Constraints to Preserve Species and Habitats
Biodiversity in Finnish Wilderness Areas: Historical and Cultural Constraints to Preserve Species and Habitats Anna-Liisa Sippola Abstract—The present status of species and habitats in Finnish wilderness areas is largely a consequence of past administrative, use, and management traditions in northern Finland. The existing wilderness legislation sets a framework for management, but historical uses and administrative decisions have influenced many prevailing practices. In addition, manage- ment of many uses is complicated by overarching legislation. The present wilderness legisla- tion is a tradeoff between conservation aspects and both traditional and modern use forms, including reindeer herding, hunting, fishing, berry picking, forestry, mineral prospecting, and tourism. Many of these use forms have negative impacts on biodiversity. Forestry, which is allowed in restricted parts of wilderness areas, fragments areas and destroys habitats of old- growth forest species. Large reindeer populations have caused overgrazing in many areas. Heavy hunting pressure has caused the decline of capercaillie and black grouse populations, and increased tourism causes disturbance of animals and terrain. The constraints to preserve species and habitats are often related to the contradictory goals of different laws or compli- cated administrative structures. Hunting is an example of a use form where different organi- zations are responsible for monitoring of game populations, making recommendations for prey numbers, selling of licences, and law enforcement. Different values and attitudes also complicate conservation efforts. Conservation of large predators, for example, conflicts with the interests of reindeer herders, often leading to poaching. This paper examines both histori- cal and cultural factors that affect the status of biodiversity in Finnish wilderness areas, and discusses possibilities to achieve commonly accepted goals and practices in biodiversity con- servation. -

Larval Parasitism of the Autumnal Moth Reduces Feeding Intensity on the Mountain Birch
Postprint version. Original publication in: Oecologia (2009) 159(3): 539-547 doi: 10.1007/s00442-008-1240-6 Larval parasitism of the autumnal moth reduces feeding intensity on the mountain birch Tea Ammunét, Netta Klemola, Annette Heisswolf, Tero Klemola Section of Ecology, Department of Biology, University of Turku, 20014 Turku, Finland Received: 18 February 2008 / Accepted: 14 November 2008 / Published online: 10 December 2008 Communicated by Richard Karban. Abstract Plants respond to grazing by herbivorous insects by emitting a range of volatile organic compounds, which attract parasitoids to their insect hosts. However, a positive outcome for the host plant is a necessary precondition for making the attraction beneficial or even adaptive. Parasitoids benefit plants by killing herbivorous insects, thus reducing future herbivore pressure, but also by curtailing the feeding intensity of the still living, par- asitised host. In this study, the effect of parasitism on food consumption of the 5th instar larvae of the autumnal moth (Epirrita autumnata) was examined under laboratory con- ditions. Daily food consumption, as well as the duration of the 5th instar, was measured for both parasitised and non-parasitised larvae. The results showed that parasitism by the solitary endoparasitoid Zele deceptor not only reduced leaf consumption significantly but also hastened the onset of pupation in autumnal moth larvae. On the basis of the results, an empirical model was derived to assess the affects on the scale of the whole tree. The model suggests that parasitoids might protect the tree from total defoliation at least at intermediate larval densities. Consequently, a potential for plant–parasitoid chemical sig- nalling appears to exist, which seems to benefit the mountain birch (Betula pubescens ssp.