The Effect of Snow on Plants and Their Interactions with Herbivores
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A REVISION of the GENUS ERIOSOMA and ITS ALLIED GENERA in JAPAN (HOMOPTERA : Title APHIDOIDEA)
A REVISION OF THE GENUS ERIOSOMA AND ITS ALLIED GENERA IN JAPAN (HOMOPTERA : Title APHIDOIDEA) Author(s) Akimoto, Shin'ichi Insecta matsumurana. New series : journal of the Faculty of Agriculture Hokkaido University, series entomology, 27, Citation 37-106 Issue Date 1983-10 Doc URL http://hdl.handle.net/2115/9821 Type bulletin (article) File Information 27_p37-106.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP INSECT A M ATSUMURANA NEW SERIES 27: 37-106 OCTOBER 1983 A REVISION OF THE GENUS ERIOSOMA AND ITS ALLIED GENERA IN JAPAN (HOMOPTERA: APHIDOIDEA) By SHIN'rcHI AKIMOTO Abstract AKIMOTO, S. 1983. A reVlSIOn of the genus Eriosoma and its allied genera in Japan (Homoptera: Aphidoidea). Ins. matsum. n.S. 27: 37-106, 3 tabs., 26 figs. Nine species of Eriosoma and its allied genera, all leaf-roll aphids on Ulmaceae and occur ring in Japan, are dealt with. Descriptions of gall-generations, exules, sexuparae and galls are given, with brief biological notes for some species. The species-complex hitherto called Eriosoma japonicum is classified into 6 species, including 5 ones new to science. A parasitic form of Eriosoma yangi, occurring on Ulmus davidiana var. japonica, is formally described as a new subspecies different from the nominate form on Ulmus parvifolia in not only behavior but also morphological characters of their fundatrices. The subdivision of Eriosoma is recon sidered in connection with the divergence and distribution of the host plants, and the ulmi group is newly proposed. A leaf-roll aphid associated with Zelkova is described as a new species belonging to Hemipodaphis, which has only been known from the morph occurring on the secon dary host in India; on the basis of the new species Hemipodaphis is transferred to the Pemphigidae (Eriosomatinae) from the Hormaphididae. -

Colonization of Ecological Islands: Galling Aphid Populations (Sternorrhyncha: Aphidoidea: Pemphigidae) on Recoveringpistacia Trees After Destruction by Fire
Eur. J. Entomol. 95: 41-53, 1998 ISSN 1210-5759 Colonization of ecological islands: Galling aphid populations (Sternorrhyncha: Aphidoidea: Pemphigidae) on recoveringPistacia trees after destruction by fire D avid WOOL and M oshe INBAR* Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, 69978 Israel; e-mail: [email protected] Gall-forming aphids, Pemphigidae, Fordinae,Pistacia, fire, recolonization Abstract. Pistacia palaestina (Anacardiaceae) is a common tree in the natural forest of Mt. Carmel, Is rael, and the primary host of five common species of gall-forming aphids (Sternorrhyncha: Aphidoidea: Pemphigidae: Fordinae). After a forest fire, resprouting P. palaestina trees, which are colonized by migrants from outside the burned area, become “ecological islands” for host-specific herbivores. A portion of the Carmel National Park was destroyed by fire in September of 1989. The same winter, thirty-nine resprouting trees that formed green islands in the otherwise barren environment were identified and marked. Tree growth was extraordinarily vigorous during the first year after the fire, but shoot elon gation declined markedly in subsequent years. Recolonization of the 39 “islands” by the Fordinae was studied for six consecutive years. Although the life cycle of the aphids and the deciduous phenology of the tree dictate that the “islands” must be newly recolonized every year, the results of this study show that trees are persistently occupied once colonized. This is probably due to establishment of aphid colonies on the roots of secondary hosts near each tree following the first successful production of a gall. Differences in colonization success of different species could be related to both the abundance of dif ferent aphid species in the unburned forest and the biological characteristics of each aphid species. -

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

Action Plan Pasvik-Inari Trilateral Park 2019-2028
Action plan Pasvik-Inari Trilateral Park 2019-2028 2019 Action plan Pasvik-Inari Trilateral Park 2019-2028 Date: 31.1.2019 Authors: Kalske, T., Tervo, R., Kollstrøm, R., Polikarpova, N. and Trusova, M. Cover photo: Young generation of birders and environmentalists looking into the future (Pasvik Zapovednik, О. Кrotova) The Trilateral Advisory Board: FIN Metsähallitus, Parks & Wildlife Finland Centre for Economic Development, Transport and the Environments in Lapland (Lapland ELY-centre) Inari Municipality NOR Office of the Finnmark County Governor Øvre Pasvik National Park Board Sør-Varanger Municipality RUS Pasvik Zapovednik Pechenga District Municipality Nikel Local Municipality Ministry of Natural Resource and Ecology of the Murmansk region Ministry of Economic Development of the Murmansk region, Tourism division Observers: WWF Barents Office Russia, NIBIO Svanhovd Norway Contacts: FINLAND NORWAY Metsähallitus, Parks & Wildlife Finland Troms and Finnmark County Governor Ivalo Customer Service Tel. +47 789 50 300 Tel. +358 205 64 7701 [email protected] [email protected] Northern Lapland Nature Centre Siida RUSSIA Tel. +358 205 64 7740 Pasvik State Nature Reserve [email protected] (Pasvik Zapovednik) Tel./fax: +7 815 54 5 07 00 [email protected] (Nikel) [email protected] (Rajakoski) 2 Action Plan Pasvik-Inari Trilateral Park 2019-2028 3 Preface In this 10-year Action Plan for the Pasvik-Inari Trilateral Park, we present the background of the long-lasting nature protection and management cooperation, our mutual vision and mission, as well as the concrete development ideas of the cooperation for the next decade. The plan is considered as an advisory plan focusing on common long-term guidance and cooperation. -

CURRICULUM VITAE Matthew P. Ayres
CURRICULUM VITAE Matthew P. Ayres Department of Biological Sciences, Dartmouth College, Hanover, NH 03755 (603) 646-2788, [email protected], http://www.dartmouth.edu/~mpayres APPOINTMENTS Professor of Biological Sciences, Dartmouth College, 2008 - Associate Director, Institute of Arctic Studies, Dartmouth College, 2014 - Associate Professor of Biological Sciences, Dartmouth College, 2000-2008 Assistant Professor of Biological Sciences, Dartmouth College, 1993 to 2000 Research Entomologist, USDA Forest Service, Research Entomologist, 1993 EDUCATION 1991 Ph.D. Entomology, Michigan State University 1986 Fulbright Fellowship, University of Turku, Finland 1985 M.S. Biology, University of Alaska Fairbanks 1983 B.S. Biology, University of Alaska Fairbanks PROFESSIONAL AFFILIATIONS Ecological Society of America Entomological Society of America PROFESSIONAL SERVICES Member, Board of Editors: Ecological Applications; Member, Editorial Board, Population Ecology Referee: (10-15 manuscripts / year) American Naturalist, Annales Zoologici Fennici, Bioscience, Canadian Entomologist, Canadian Journal of Botany, Canadian Journal of Forest Research, Climatic Change, Ecography, Ecology, Ecology Letters, Ecological Entomology, Ecological Modeling, Ecoscience, Environmental Entomology, Environmental & Experimental Botany, European Journal of Entomology, Field Crops Research, Forest Science, Functional Ecology, Global Change Biology, Journal of Applied Ecology, Journal of Biogeography, Journal of Geophysical Research - Biogeosciences, Journal of Economic -

POPULATION DYNAMICS of the SYCAMORE APHID (Drepanosiphum Platanoidis Schrank)
POPULATION DYNAMICS OF THE SYCAMORE APHID (Drepanosiphum platanoidis Schrank) by Frances Antoinette Wade, B.Sc. (Hons.), M.Sc. A thesis submitted for the degree of Doctor of Philosophy of the University of London, and the Diploma of Imperial College of Science, Technology and Medicine. Department of Biology, Imperial College at Silwood Park, Ascot, Berkshire, SL5 7PY, U.K. August 1999 1 THESIS ABSTRACT Populations of the sycamore aphid Drepanosiphum platanoidis Schrank (Homoptera: Aphididae) have been shown to undergo regular two-year cycles. It is thought this phenomenon is caused by an inverse seasonal relationship in abundance operating between spring and autumn of each year. It has been hypothesised that the underlying mechanism of this process is due to a plant factor, intra-specific competition between aphids, or a combination of the two. This thesis examines the population dynamics and the life-history characteristics of D. platanoidis, with an emphasis on elucidating the factors involved in driving the dynamics of the aphid population, especially the role of bottom-up forces. Manipulating host plant quality with different levels of aphids in the early part of the year, showed that there was a contrast in aphid performance (e.g. duration of nymphal development, reproductive duration and output) between the first (spring) and the third (autumn) aphid generations. This indicated that aphid infestation history had the capacity to modify host plant nutritional quality through the year. However, generalist predators were not key regulators of aphid abundance during the year, while the specialist parasitoids showed a tightly bound relationship to its prey. The effect of a fungal endophyte infecting the host plant generally showed a neutral effect on post-aestivation aphid dynamics and the degree of parasitism in autumn. -

Causes of Cyclicity of Epirrita Autumnata (Lepidoptera, Geometridae): Grandiose Theory and Tedious Practice
Popul Ecol (2000) 42:211–223 © The Society of Population Ecology and Springer-Verlag Tokyo 2000 SPECIAL FEATURE: REVIEW Kai Ruohomäki · Miia Tanhuanpää · Matthew P. Ayres Pekka Kaitaniemi · Toomas Tammaru · Erkki Haukioja Causes of cyclicity of Epirrita autumnata (Lepidoptera, Geometridae): grandiose theory and tedious practice Received: April 6, 1999 / Accepted: April 18, 2000 Abstract Creating multiyear cycles in population density Key words Cyclic population dynamics · Host plant quality demands, in traditional models, causal factors that operate · Inducible resistance · Parasitism · Predation · Winter on local populations in a density-dependent way with time mortality lags. However, cycles of the geometrid Epirrita autumnata in northern Europe may be regional, not local; i.e., succes- sive outbreaks occur in different localities. We review pos- sible causes of cycles of E. autumnata under both local and regional scenarios, including large-scale synchrony. Assum- Introduction ing cyclicity is a local phenomenon, individual populations of E. autumnata display peaks but populations all over the Most natural populations of herbivorous insects do not outbreak range fluctuate in synchrony. This concept as- reach outbreak densities (Mattson and Addy 1975; Mason sumes that the peaks at most localities are so low that they 1987; Hunter 1995) while a few others (Baltensweiler et al. do not lead to visible defoliation and easily remain unno- 1977; Ginzburg and Taneyhill 1994; Myers 1993; Berryman ticed. In this scenario, populations are able to start recovery 1995) display irregular outbreaks or regular cycles, an out- a few years after the crash, i.e., at the time of the mitigation break being defined as an “explosive increase in the abun- of detrimental delayed density-dependent factors, such as dance of a particular species that occurs over a relatively delayed inducible resistance of the host plant or parasitism. -

What Causes Population Cycles of Forest Lepidoptera?
PERSPECTIVES to track numerical changes in their preyg. During the writing of this article, Werner What causes population cycles of forest Baltensweiler (Swiss Federal Institute of Technology, retired) sent me data on the Lepidoptera? two dominant parasitoids attacking the larch budmoth. Theoretical model-fitting (Box 1) indicates that the parasite-bud- Alan A. Berryman percapita rate of increase, R = ln(N,/N,-,), worm interaction only explains 28% of the and population density in the previous observed per-capita growth rate of the year, In N,-z, from which the effect of first- budworm and 50% of the parasitoids, Hypotheses for the causes of order correlation between R and In N,-, has which is hardly a dominant effect. regular cycles in populations of been removed. Lepidoptera with cyclical There seems to be little doubt, how- forest Lepidoptera have invoked dynamics are dominated by second-order ever, that population cycles of some for- pathogen-insect or foliage-insect feedback (PC2 > Kl) (see Fig. 1) while est Lepidoptera are the result of interac- interactions. However, the available those with more stable dynamics are domi- tions with insect parasitoids. For example, data suggest that forest caterpillar nated by first-order feedback (PC2 <Xl). Morris6 presented 11 years of data on the cycles are more likely to be the result The search for a general explanation density of blackheaded budworm (Acleris of interactions with insect parasitoids, for population cycles in forest Lepidop- UQriQnQ) caterpillars and their larval para- an old argument that seems to have tera has been approached from two major sitoids and concluded that the dynamics been neglected in recent years. -
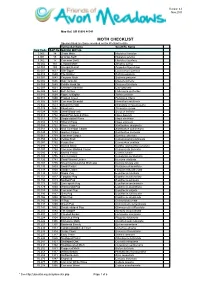
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Interactions Between a Leaf-Galling Wasp and Its Invasive Hawkweed Hosts
INTERACTIONS BETWEEN A LEAF-GALLING WASP AND ITS INVASIVE HAWKWEED HOSTS GREGORY HOLMES Bachelor of Science, University of Lethbridge, 2013 A Thesis Submitted to the School of Graduate Studies of the University of Lethbridge in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE Department of Biological Sciences University of Lethbridge LETHBRIDGE, ALBERTA, CANADA © Gregory Holmes, 2016 INTERACTIONS BETWEEN A LEAF-GALLING WASP AND ITS INVASIVE HAWKWEED HOST GREGORY HOLMES Date of Defence: June 20, 2016 Rosemarie De Clerck-Floate Research Scientist Ph.D. Co-supervisor Robert Laird Associate Professor Ph.D. Co-supervisor Syama Chatterton Research Scientist Ph.D. Committee Member Cameron Goater Professor Ph.D. Committee Member Theresa Burg Associate Professor Ph.D. Chair, Thesis Examination Committee I would like to dedicate this thesis to my wonderful wife, Jessica, and to my parents. Their unfailing support has allowed me to pursue my dreams in all of life's endeavors. iii Abstract This thesis aims to explore the interactions between a potential biocontrol agent, the gall-wasp Aulacidea pilosellae, and its invasive Pilosella hawkweed hosts. I discovered that increased nitrogen availability improves P. officinarum vegetative growth, while also interacting with A. pilosellae to reduce vegetative growth, as the plants cannot compensate for this herbivory. I did not detect any nitrogen effects on wasp performance. I also explored how the host species utilized for galling affects wasp performance through two generations by measuring maternal effects. These only influenced offspring performance when the mother had utilized P. caespitosa, but not P. glomerata. I discovered that P. caespitosa is also the better offspring host, producing significantly larger galls and heavier larvae compared to P. -
Taxonomic Study on Gall Aphids, Colopha
TAXONOMIC STUDY ON GALL APHIDS, COLOPHA, PARACOLOPHA AND KALTENBACHIELLA Title (APHIDOIDEA : PEMPHIGIDAE) IN EAST ASIA, WITH SPECIAL REFERENCE TO THEIR ORIGINS AND DISTRIBUTIONAL PATTERNS Author(s) Akimoto, Shin'ichi Insecta matsumurana. New series : journal of the Faculty of Agriculture Hokkaido University, series entomology, 31, 1- Citation 79 Issue Date 1985-03 Doc URL http://hdl.handle.net/2115/9827 Type bulletin (article) File Information 31_p1-79.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP INSECTA MATSUMURANA NEW SERIES 31: 1-79 MARCH 1985 TAXONOMIC STUDY ON GALL APHIDS, COLOPHA, PARACOLOPHA AND KALTENBACHIELLA (APHIDOIDEA: PEMPHIGIDAE) IN EAST ASIA, WITH SPECIAL REFERENCE TO THEIR ORIGINS AND DISTRIBUTIONAL PATTERNS By SHIN-ICHI AKIMOTO Abstract AKIMOTO, S. 1985. Taxonomic study on gall aphids, Colopha, Paracolopha and Kalten· bachiella (Aphidoidea: Pemphigidae) in East Asia, with special reference to their origins and distributional patterns. Ins. matsum. n. s. 31: 1-79,27 tabs., 32 figs. (28 text·figs., 4 pis.). Eight East Asian species of the tribe Tetraneurini (exclusive of Tetraneura) are revised with remarks on their biology and distribution. Colopha moriokaensis (Monzen, 1923), a common gall aphid occurring on Zelkova serrata, is synonymized with Paracolopha morrisoni (Baker, 1919) known from North America, and Paracolopha takahashii sp. nov. is described from Japan. Based on this treatment the Tetraneurini are to include Paracolopha in addition to Colopha, Kaltenbachiella and Tetraneura. Colopha graminis (Takahashi, 1930) is synonymized with C. kansugei (Uye, 1924), which is shown to have a wide range from East Asia to Nepal. Kaltenbachiella spinosa sp. nov. is distinguished from K. -

Chemistry& Metabolism Chemical Information National Library Of
Chemistry& Metabolism Chemical Information National Library of Medicine Chemical Resources of the Environmental Health & Toxicology Information Program chemistry.org: American Chemical Society - ACS HomePage Identified Compounds — Metabolomics Fiehn Lab AOCS > Publications > Online Catalog > Modern Methods for Lipid Analysis by Liquid Chromatography/Mass Spectrometry and Related Techniques (Lipase Database) Lipid Library Lipid Library Grom Analytik + HPLC GmbH: Homepage Fluorescence-based phospholipase assays—Table 17,3 | Life Technologies Phosphatidylcholine | PerkinElmer MetaCyc Encyclopedia of Metabolic Pathways MapMan Max Planck Institute of Molecular Plant Physiology MS analysis MetFrag Scripps Center For Metabolomics and Mass Spectrometry - XCMS MetaboAnalyst Lipid Analysis with GC-MS, LC-MS, FT-MS — Metabolomics Fiehn Lab MetLIn LOX and P450 inhibitors Lipoxygenase inhibitor BIOMOL International, LP - Lipoxygenase Inhibitors Lipoxygenase structure Lypoxygenases Lipoxygenase structure Plant databases (see also below) PlantsDB SuperSAGE & SAGE Serial Analysis of Gene Expression: Information from Answers.com Oncology: The Sidney Kimmel Comprehensive Cancer Center EMBL Heidelberg - The European Molecular Biology Laboratory EMBL - SAGE for beginners Human Genetics at Johns Hopkins - Kinzler, K Serial Analysis of Gene Expression The Science Creative Quarterly » PAINLESS GENE EXPRESSION PROFILING: SAGE (SERIAL ANALYSIS OF GENE EXPRESSION) IDEG6 software home page (Analysis of gene expression) GenXPro :: GENome-wide eXpression PROfiling