Maternal Effects on Egg Numbers of the Autumnal Moth Under Field Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Antonino De Souza Jr JD, Et Al.(2013) Transcriptome Analysis in Cotton
Transcriptome Analysis in Cotton Boll Weevil (Anthonomus grandis) and RNA Interference in Insect Pests Alexandre Augusto Pereira Firmino1,2*☯, Fernando Campos de Assis Fonseca1,3☯, Leonardo Lima Pepino de Macedo1,4☯, Roberta Ramos Coelho1,3, José Dijair Antonino de Souza Jr1,3, Roberto Coiti Togawa1, Orzenil Bonfim Silva-Junior1, Georgios Joannis Pappas-Jr3, Maria Cristina Mattar da Silva1, Gilbert Engler5, Maria Fatima Grossi-de-Sa1,4* 1 Embrapa Recursos Genéticos e Biotecnologia, Brasília, Distrito Federal, Brazil, 2 Graduate Program in Cellular and Molecular Biology, Center of Biotechnology, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil, 3 Graduate Program in Biology Molecular, Universidade de Brasília, Brasília, Distrito Federal, Brazil, 4 Graduate Program in Genomic Sciences and Biotechnology, Universidade Católica de Brasília, Brasília, Distrito Federal, Brazil, 5 Plateau Microscopique, Institut National de la Recherche Agronomique, Sophia-Antipolis, France Abstract Cotton plants are subjected to the attack of several insect pests. In Brazil, the cotton boll weevil, Anthonomus grandis, is the most important cotton pest. The use of insecticidal proteins and gene silencing by interference RNA (RNAi) as techniques for insect control are promising strategies, which has been applied in the last few years. For this insect, there are not much available molecular information on databases. Using 454-pyrosequencing methodology, the transcriptome of all developmental stages of the insect pest, A. grandis, was analyzed. The A. grandis transcriptome analysis resulted in more than 500.000 reads and a data set of high quality 20,841 contigs. After sequence assembly and annotation, around 10,600 contigs had at least one BLAST hit against NCBI non- redundant protein database and 65.7% was similar to Tribolium castaneum sequences. -

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

A Comprehensive DNA Barcode Library for the Looper Moths (Lepidoptera: Geometridae) of British Columbia, Canada
AComprehensiveDNABarcodeLibraryfortheLooper Moths (Lepidoptera: Geometridae) of British Columbia, Canada Jeremy R. deWaard1,2*, Paul D. N. Hebert3, Leland M. Humble1,4 1 Department of Forest Sciences, University of British Columbia, Vancouver, British Columbia, Canada, 2 Entomology, Royal British Columbia Museum, Victoria, British Columbia, Canada, 3 Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario, Canada, 4 Canadian Forest Service, Natural Resources Canada, Victoria, British Columbia, Canada Abstract Background: The construction of comprehensive reference libraries is essential to foster the development of DNA barcoding as a tool for monitoring biodiversity and detecting invasive species. The looper moths of British Columbia (BC), Canada present a challenging case for species discrimination via DNA barcoding due to their considerable diversity and limited taxonomic maturity. Methodology/Principal Findings: By analyzing specimens held in national and regional natural history collections, we assemble barcode records from representatives of 400 species from BC and surrounding provinces, territories and states. Sequence variation in the barcode region unambiguously discriminates over 93% of these 400 geometrid species. However, a final estimate of resolution success awaits detailed taxonomic analysis of 48 species where patterns of barcode variation suggest cases of cryptic species, unrecognized synonymy as well as young species. Conclusions/Significance: A catalog of these taxa meriting further taxonomic investigation is presented as well as the supplemental information needed to facilitate these investigations. Citation: deWaard JR, Hebert PDN, Humble LM (2011) A Comprehensive DNA Barcode Library for the Looper Moths (Lepidoptera: Geometridae) of British Columbia, Canada. PLoS ONE 6(3): e18290. doi:10.1371/journal.pone.0018290 Editor: Sergios-Orestis Kolokotronis, American Museum of Natural History, United States of America Received August 31, 2010; Accepted March 2, 2011; Published March 28, 2011 Copyright: ß 2011 deWaard et al. -

Datana Drexelii (Lepidoptera: Notododontidae) Occurrence and Larval Survival on Highbush Blueberry Cultivars
University of Rhode Island DigitalCommons@URI Biological Sciences Faculty Publications Biological Sciences 2020 Datana drexelii (Lepidoptera: Notododontidae) occurrence and larval survival on highbush blueberry cultivars Alex K. Baranowski University of Rhode Island Steven R. Alm University of Rhode Island, [email protected] Evan L. Preisser University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/bio_facpubs The University of Rhode Island Faculty have made this article openly available. Please let us know how Open Access to this research benefits you. This is a pre-publication author manuscript of the final, published article. Terms of Use This article is made available under the terms and conditions applicable towards Open Access Policy Articles, as set forth in our Terms of Use. Citation/Publisher Attribution Baranowski, A.K., Alm, S.R., and E.L. Preisser. 2020. Datana drexelii (Lepidoptera: Notododontidae) oviposition and larval survival on highbush blueberry cultivars. Journal of Economic Entomology, 113(3), 1568-1571. doi: 10.1093/jee/toaa050 Available at: https://doi.org/10.1093/jee/toaa050 This Article is brought to you for free and open access by the Biological Sciences at DigitalCommons@URI. It has been accepted for inclusion in Biological Sciences Faculty Publications by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. 1 1 Alex Baranowski 2 Department of Biological Sciences 3 University of Rhode Island 4 Woodward Hall 5 9 East Alumni Avenue 6 Kingston, RI 02881 USA 7 (860) 378-7430 8 [email protected] 9 10 11 Datana drexelii (Lepidoptera: Notododontidae) occurrence and larval survival on highbush 12 blueberry cultivars 13 14 ALEX K. -

Action Plan Pasvik-Inari Trilateral Park 2019-2028
Action plan Pasvik-Inari Trilateral Park 2019-2028 2019 Action plan Pasvik-Inari Trilateral Park 2019-2028 Date: 31.1.2019 Authors: Kalske, T., Tervo, R., Kollstrøm, R., Polikarpova, N. and Trusova, M. Cover photo: Young generation of birders and environmentalists looking into the future (Pasvik Zapovednik, О. Кrotova) The Trilateral Advisory Board: FIN Metsähallitus, Parks & Wildlife Finland Centre for Economic Development, Transport and the Environments in Lapland (Lapland ELY-centre) Inari Municipality NOR Office of the Finnmark County Governor Øvre Pasvik National Park Board Sør-Varanger Municipality RUS Pasvik Zapovednik Pechenga District Municipality Nikel Local Municipality Ministry of Natural Resource and Ecology of the Murmansk region Ministry of Economic Development of the Murmansk region, Tourism division Observers: WWF Barents Office Russia, NIBIO Svanhovd Norway Contacts: FINLAND NORWAY Metsähallitus, Parks & Wildlife Finland Troms and Finnmark County Governor Ivalo Customer Service Tel. +47 789 50 300 Tel. +358 205 64 7701 [email protected] [email protected] Northern Lapland Nature Centre Siida RUSSIA Tel. +358 205 64 7740 Pasvik State Nature Reserve [email protected] (Pasvik Zapovednik) Tel./fax: +7 815 54 5 07 00 [email protected] (Nikel) [email protected] (Rajakoski) 2 Action Plan Pasvik-Inari Trilateral Park 2019-2028 3 Preface In this 10-year Action Plan for the Pasvik-Inari Trilateral Park, we present the background of the long-lasting nature protection and management cooperation, our mutual vision and mission, as well as the concrete development ideas of the cooperation for the next decade. The plan is considered as an advisory plan focusing on common long-term guidance and cooperation. -

CURRICULUM VITAE Matthew P. Ayres
CURRICULUM VITAE Matthew P. Ayres Department of Biological Sciences, Dartmouth College, Hanover, NH 03755 (603) 646-2788, [email protected], http://www.dartmouth.edu/~mpayres APPOINTMENTS Professor of Biological Sciences, Dartmouth College, 2008 - Associate Director, Institute of Arctic Studies, Dartmouth College, 2014 - Associate Professor of Biological Sciences, Dartmouth College, 2000-2008 Assistant Professor of Biological Sciences, Dartmouth College, 1993 to 2000 Research Entomologist, USDA Forest Service, Research Entomologist, 1993 EDUCATION 1991 Ph.D. Entomology, Michigan State University 1986 Fulbright Fellowship, University of Turku, Finland 1985 M.S. Biology, University of Alaska Fairbanks 1983 B.S. Biology, University of Alaska Fairbanks PROFESSIONAL AFFILIATIONS Ecological Society of America Entomological Society of America PROFESSIONAL SERVICES Member, Board of Editors: Ecological Applications; Member, Editorial Board, Population Ecology Referee: (10-15 manuscripts / year) American Naturalist, Annales Zoologici Fennici, Bioscience, Canadian Entomologist, Canadian Journal of Botany, Canadian Journal of Forest Research, Climatic Change, Ecography, Ecology, Ecology Letters, Ecological Entomology, Ecological Modeling, Ecoscience, Environmental Entomology, Environmental & Experimental Botany, European Journal of Entomology, Field Crops Research, Forest Science, Functional Ecology, Global Change Biology, Journal of Applied Ecology, Journal of Biogeography, Journal of Geophysical Research - Biogeosciences, Journal of Economic -

Phylogenetic Relationships of the Tribe Operophterini (Lepidoptera, Geometridae): a Case Study of the Evolution of Female flightlessness
Biological Journal of the Linnean Society, 2007, 92, 241–252. With 1 figure Phylogenetic relationships of the tribe Operophterini (Lepidoptera, Geometridae): a case study of the evolution of female flightlessness NIINA SNÄLL1,2, TOOMAS TAMMARU4*, NIKLAS WAHLBERG1,5, JAAN VIIDALEPP6, KAI RUOHOMÄKI2, MARJA-LIISA SAVONTAUS1 and KIRSI HUOPONEN3 1Laboratory of Genetics, Department of Biology, 2Section of Ecology, Department of Biology, and 3Department of Medical Genetics, University of Turku, FIN-20014 Turku, Finland 4Institute of Zoology and Hydrobiology, University of Tartu, Vanemuise 46, EE-51014 Tartu, Estonia 5Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 6Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Kreutzwaldi 64, EE-51014 Tartu, Estonia Received 24 April 2006; accepted for publication 20 November 2006 A molecular phylogenetic analysis was conducted in order to reconstruct the evolution of female flightlessness in the geometrid tribe Operophterini (Lepidoptera, Geometridae, Larentiinae). DNA variation in four nuclear gene regions, segments D1 and D2 of 28S rRNA, elongation factor 1a, and wingless, was examined from 22 species representing seven tribes of Larentiinae and six outgroup species. Direct optimization was used to infer a phylogenetic hypothesis from the combined sequence data set. The results obtained confirmed that Operophterini (including Malacodea) is a monophyletic group, and Perizomini is its sister group. Within Operophterini, the genus Malacodea is the sister group to the genera Operophtera and Epirrita, which form a monophyletic group. This relationship is also supported by morphological data. The results suggest that female flightlessness has evolved independently twice: first in the lineage of Malacodea and, for the second time, in the lineage of Operophtera after its separation from the lineage of Epirrita. -

Causes of Cyclicity of Epirrita Autumnata (Lepidoptera, Geometridae): Grandiose Theory and Tedious Practice
Popul Ecol (2000) 42:211–223 © The Society of Population Ecology and Springer-Verlag Tokyo 2000 SPECIAL FEATURE: REVIEW Kai Ruohomäki · Miia Tanhuanpää · Matthew P. Ayres Pekka Kaitaniemi · Toomas Tammaru · Erkki Haukioja Causes of cyclicity of Epirrita autumnata (Lepidoptera, Geometridae): grandiose theory and tedious practice Received: April 6, 1999 / Accepted: April 18, 2000 Abstract Creating multiyear cycles in population density Key words Cyclic population dynamics · Host plant quality demands, in traditional models, causal factors that operate · Inducible resistance · Parasitism · Predation · Winter on local populations in a density-dependent way with time mortality lags. However, cycles of the geometrid Epirrita autumnata in northern Europe may be regional, not local; i.e., succes- sive outbreaks occur in different localities. We review pos- sible causes of cycles of E. autumnata under both local and regional scenarios, including large-scale synchrony. Assum- Introduction ing cyclicity is a local phenomenon, individual populations of E. autumnata display peaks but populations all over the Most natural populations of herbivorous insects do not outbreak range fluctuate in synchrony. This concept as- reach outbreak densities (Mattson and Addy 1975; Mason sumes that the peaks at most localities are so low that they 1987; Hunter 1995) while a few others (Baltensweiler et al. do not lead to visible defoliation and easily remain unno- 1977; Ginzburg and Taneyhill 1994; Myers 1993; Berryman ticed. In this scenario, populations are able to start recovery 1995) display irregular outbreaks or regular cycles, an out- a few years after the crash, i.e., at the time of the mitigation break being defined as an “explosive increase in the abun- of detrimental delayed density-dependent factors, such as dance of a particular species that occurs over a relatively delayed inducible resistance of the host plant or parasitism. -

What Causes Population Cycles of Forest Lepidoptera?
PERSPECTIVES to track numerical changes in their preyg. During the writing of this article, Werner What causes population cycles of forest Baltensweiler (Swiss Federal Institute of Technology, retired) sent me data on the Lepidoptera? two dominant parasitoids attacking the larch budmoth. Theoretical model-fitting (Box 1) indicates that the parasite-bud- Alan A. Berryman percapita rate of increase, R = ln(N,/N,-,), worm interaction only explains 28% of the and population density in the previous observed per-capita growth rate of the year, In N,-z, from which the effect of first- budworm and 50% of the parasitoids, Hypotheses for the causes of order correlation between R and In N,-, has which is hardly a dominant effect. regular cycles in populations of been removed. Lepidoptera with cyclical There seems to be little doubt, how- forest Lepidoptera have invoked dynamics are dominated by second-order ever, that population cycles of some for- pathogen-insect or foliage-insect feedback (PC2 > Kl) (see Fig. 1) while est Lepidoptera are the result of interac- interactions. However, the available those with more stable dynamics are domi- tions with insect parasitoids. For example, data suggest that forest caterpillar nated by first-order feedback (PC2 <Xl). Morris6 presented 11 years of data on the cycles are more likely to be the result The search for a general explanation density of blackheaded budworm (Acleris of interactions with insect parasitoids, for population cycles in forest Lepidop- UQriQnQ) caterpillars and their larval para- an old argument that seems to have tera has been approached from two major sitoids and concluded that the dynamics been neglected in recent years. -
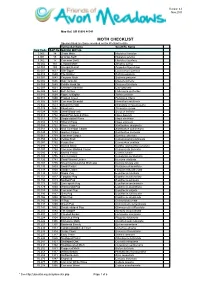
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Local Outbreaks of Operophtera Brumata and Operophtera Fagata Cannot Be Explained by Low Vulnerability to Pupal Predation
Postprint version. Original publication in: Agricultural and Forest Entomology (2010) 12(1): 81-87 doi: 10.1111/j.1461-9563.2009.00455.x Local outbreaks of Operophtera brumata and Operophtera fagata cannot be explained by low vulnerability to pupal predation Annette Heisswolf, Miia Käär, Tero Klemola, Kai Ruohomäki Section of Ecology, Department of Biology, University of Turku, FI-20014 Turku, Finland Abstract. One of the unresolved questions in studies on population dynamics of for- est Lepidoptera is why some populations at times reach outbreak densities, whereas oth- ers never do. Resolving this question is especially challenging if populations of the same species in different areas or of closely-related species in the same area are considered. The present study focused on three closely-related geometrid moth species, autumnal Epirrita autumnata, winter Operophtera brumata and northern winter moths Operophtera fagata, in southern Finland. There, winter and northern winter moth populations can reach outbreak densities, whereas autumnal moth densities stay relatively low. We tested the hypothesis that a lower vulnerability to pupal predation may explain the observed differences in population dynamics. The results obtained do not support this hypothesis because pupal predation probabilities were not significantly different between the two genera within or without the Operophtera outbreak area or in years with or without a current Operophtera outbreak. Overall, pupal predation was even higher in winter and northern winter moths than in autumnal moths. Differences in larval predation and parasitism, as well as in the repro- ductive capacities of the species, might be other candidates. Keywords. Epirrita autumnata; forest Lepidoptera; Operophtera brumata; Operoph- tera fagata; outbreak; population dynamics; pupal predation.