Winter Geometrid Moths in Oak Forests: Is Monitoring a Single Species Reliable to Predict Defoliation Risk?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A New Macrolepidopteran Moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican Amber
ZooKeys 965: 73–84 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.965.54461 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber Weiting Zhang1,2, Chungkun Shih3,4, YuHong Shih5, Dong Ren3 1 Hebei GEO University, 136 Huaiandonglu, Shijiazhuang 050031, China 2 State Key Laboratory of Pal- aeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, CAS, Nanjing 210008, China 3 College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, 105 Xisan- huanbeilu, Haidian District, Beijing 100048, China 4 Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, USA 5 Laboratorio Dominicano De Ambar Y Gemas, Santo Domingo, Dominican Republic Corresponding author: Weiting Zhang ([email protected]) Academic editor: Gunnar Brehm | Received 19 May 2020 | Accepted 12 August 2020 | Published 3 September 2020 http://zoobank.org/05E273DB-B590-42D1-8234-864A787BE6A0 Citation: Zhang W, Shih C, Shih YH, Ren D (2020) A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber. ZooKeys 965: 73–84. https://doi.org/10.3897/zookeys.965.54461 Abstract A new genus and species of fossil moth, Miogeometrida chunjenshihi Zhang, Shih & Shih, gen. et sp. nov., assigned to Geometridae, is described from Miocene Dominican amber dating from 15–20 Mya. The new genus is characterized by the forewing without a fovea, R1 not anastomosing with Sc, no areole formed by veins R1 and Rs, R1 and Rs1 completely coincident, M2 arising midway between M1 and M3, anal veins 1A and 2A fused for their entire lengths; and the hind wing with Rs running close to Sc + R1 and M2 absent. -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Seasonal Changes in Lipid and Fatty Acid Profiles of Sakarya
Eurasian Journal of Forest Science ISSN: 2147 - 7493 Copyrights Eurasscience Journals Editor in Chief Hüseyin Barış TECİMEN University of Istanbul, Faculty of Forestry, Soil Science and Ecology Dept. İstanbul, Türkiye Journal Cover Design Mert EKŞİ Istanbul University Faculty of Forestry Department of Landscape Techniques Bahçeköy-Istanbul, Turkey Technical Advisory Osman Yalçın YILMAZ Surveying and Cadastre Department of Forestry Faculty of Istanbul University, 34473, Bahçeköy, Istanbul-Türkiye Cover Page Bolu forests, Turkey 2019 Ufuk COŞGUN Contact H. Barış TECİMEN Istanbul University-Cerrahpasa, Faculty of Forestry, Soil Science and Ecology Dept. İstanbul, Turkey [email protected] Journal Web Page http://dergipark.gov.tr/ejejfs Eurasian Journal of Forest Science Eurasian Journal of Forest Science is published 3 times per year in the electronic media. This journal provides immediate open access to its content on the principle that making research freely available to the public supports a greater global exchange of knowledge. In submitting the manuscript, the authors certify that: They are authorized by their coauthors to enter into these arrangements. The work described has not been published before (except in the form of an abstract or as part of a published lecture, review or thesis), that it is not under consideration for publication elsewhere, that its publication has been approved by all the authors and by the responsible authorities tacitly or explicitly of the institutes where the work has been carried out. They secure the right to reproduce any material that has already been published or copyrighted elsewhere. The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party. -

Contributions to Knowledge of the Geometrid Fauna of Bulgaria and Greece, with Four Species New for the Greek Fauna (Lepidoptera: Geometridae) (Plate 12)
Esperiana Band 18: 221- 224 Bad Staffelstein; Schwanfeld, 02. Dezember 2013 ISBN 978-3-938249-04-8 Contributions to knowledge of the geometrid fauna of Bulgaria and Greece, with four species new for the Greek fauna (Lepidoptera: Geometridae) (plate 12) Balázs TÓTH, János BABICS & Balázs BENEDEK Abstract During a tour led by the authors to Bulgaria and Greece in March, 2013, a total of 19 geometrid species were observed at five localities. Biston achyra WEHRLI, 1936, Agriopis marginaria (FABRICIUS, 1776), A. leucophaearia ([DENIS & SCHIffERMÜLLER], 1775) and Erannis ankeraria (STAUDINGER, 1861) were found for the first time in Greece. An entirely new habitat type is included to the biotope range of E. ankeraria, arousing the possibility of this species being widespread in the Mediterranean countries. The authors hope that these observations will encourage attention to the exploration of the populations, thereby contributing to the more efficient protection of this species. Checklists are given to each collecting events. Key words: Biston, Agriopis, Erannis, Bulgaria, Greece, new data, oak woodland, macchia-scrub, soil types Introduction Biston achyra WEHRLI, 1936 was described from Asia Minor and subsequently found in Ukraine (KOSTJUK, 1990), the Levant (KOSTJUK, 1991) and Russia (SINEV, 2008). This species can be distinguished from its relative B. strataria (HUFNAGEL, 1767) by its considerably smaller size, more elongated forewing, and the presence of discal spot on the hindwing. Agriopis marginaria (FABRICIUS, 1776) and A. leucophaearia ([DENIS & SCHIFFERMÜLLER], 1775) are both frequent and widespread in Europe. The former species is distributed from the Iberian Peninsula to the Urals and the Caucasus Mts., and is also present in Asia Minor. -

Iridopsis Socoromaensis Sp. N., a Geometrid Moth (Lepidoptera, Geometridae) from the Andes of Northern Chile
Biodiversity Data Journal 9: e61592 doi: 10.3897/BDJ.9.e61592 Taxonomic Paper Iridopsis socoromaensis sp. n., a geometrid moth (Lepidoptera, Geometridae) from the Andes of northern Chile Héctor A. Vargas ‡ ‡ Universidad Tarapacá, Arica, Chile Corresponding author: Héctor A. Vargas ([email protected]) Academic editor: Axel Hausmann Received: 02 Dec 2020 | Accepted: 26 Jan 2021 | Published: 28 Jan 2021 Citation: Vargas HA (2021) Iridopsis socoromaensis sp. n., a geometrid moth (Lepidoptera, Geometridae) from the Andes of northern Chile. Biodiversity Data Journal 9: e61592. https://doi.org/10.3897/BDJ.9.e61592 ZooBank: urn:lsid:zoobank.org:pub:3D37F554-E2DC-443C-B11A-8C7E32D88F4F Abstract Background Iridopsis Warren, 1894 (Lepidoptera: Geometridae: Ennominae: Boarmiini) is a New World moth genus mainly diversified in the Neotropical Region. It is represented in Chile by two described species, both from the Atacama Desert. New information Iridopsis socoromaensis sp. n. (Lepidoptera: Geometridae: Ennominae: Boarmiini) is described and illustrated from the western slopes of the Andes of northern Chile. Its larvae were found feeding on leaves of the Chilean endemic shrub Dalea pennellii (J.F. Macbr.) J.F. Macbr. var. chilensis Barneby (Fabaceae). Morphological differences of I. socoromaensis sp. n. with the two species of the genus previously known from Chile are discussed. A DNA barcode fragment of I. socoromaensis sp. n. showed 93.7-94.3% similarity with the Nearctic I. sanctissima (Barnes & McDunnough, 1917). However, the morphology of the genitalia suggests that these two species are distantly related. The © Vargas H. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Poplars and Willows: Trees for Society and the Environment / Edited by J.G
Poplars and Willows Trees for Society and the Environment This volume is respectfully dedicated to the memory of Victor Steenackers. Vic, as he was known to his friends, was born in Weelde, Belgium, in 1928. His life was devoted to his family – his wife, Joanna, his 9 children and his 23 grandchildren. His career was devoted to the study and improve- ment of poplars, particularly through poplar breeding. As Director of the Poplar Research Institute at Geraardsbergen, Belgium, he pursued a lifelong scientific interest in poplars and encouraged others to share his passion. As a member of the Executive Committee of the International Poplar Commission for many years, and as its Chair from 1988 to 2000, he was a much-loved mentor and powerful advocate, spreading scientific knowledge of poplars and willows worldwide throughout the many member countries of the IPC. This book is in many ways part of the legacy of Vic Steenackers, many of its contributing authors having learned from his guidance and dedication. Vic Steenackers passed away at Aalst, Belgium, in August 2010, but his work is carried on by others, including mem- bers of his family. Poplars and Willows Trees for Society and the Environment Edited by J.G. Isebrands Environmental Forestry Consultants LLC, New London, Wisconsin, USA and J. Richardson Poplar Council of Canada, Ottawa, Ontario, Canada Published by The Food and Agriculture Organization of the United Nations and CABI CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © FAO, 2014 FAO encourages the use, reproduction and dissemination of material in this information product. -

Download (236Kb)
Chapter (non-refereed) Welch, R.C.. 1981 Insects on exotic broadleaved trees of the Fagaceae, namely Quercus borealis and species of Nothofagus. In: Last, F.T.; Gardiner, A.S., (eds.) Forest and woodland ecology: an account of research being done in ITE. Cambridge, NERC/Institute of Terrestrial Ecology, 110-115. (ITE Symposium, 8). Copyright © 1981 NERC This version available at http://nora.nerc.ac.uk/7059/ NERC has developed NORA to enable users to access research outputs wholly or partially funded by NERC. Copyright and other rights for material on this site are retained by the authors and/or other rights owners. Users should read the terms and conditions of use of this material at http://nora.nerc.ac.uk/policies.html#access This document is extracted from the publisher’s version of the volume. If you wish to cite this item please use the reference above or cite the NORA entry Contact CEH NORA team at [email protected] 110 The fauna, including pests, of woodlands and forests 25. INSECTS ON EXOTIC BROADLEAVED jeopardize Q. borealis here. Moeller (1967) suggest- TREES OF THE FAGACEAE, NAMELY ed that the general immunity of Q. borealis to QUERCUS BOREALIS AND SPECIES OF insect attack in Germany was due to its planting in NOTHOFAGUS mixtures with other Quercus spp. However, its defoliation by Tortrix viridana (green oak-roller) was observed in a relatively pure stand of 743 R.C. WELCH hectares. More recently, Zlatanov (1971) published an account of insect pests on 7 species of oak in Bulgaria, including Q. rubra (Table 35), although Since the early 1970s, and following a number of his lists include many pests not known to occur earlier plantings, it seems that American red oak in Britain. -

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

(Geometridae) in Oak Forests from Romania
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Annals of the University of Craiova - Agriculture, Montanology, Cadastre Series Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies) Voll. XLIV 2014 EVOLUTION OF INFESTATIONS WITH LOOPERMOTH (GEOMETRIDAE) IN OAK FORESTS FROM ROMANIA AUTHORS: Neţoiu Constantin, Univ. Craiova Tomescu Romică, ICAS Bucureşti Vladescu Dumitru, RNP - Romsilva Aldea Dan Ioan, RNP - Romsilva Buzatu Andrei, ICAS Craiova Keywords: Geometridae, Operophtera brumata, outbreaks, infestation. ABSTRACT In the oak forests from Romania, the species of Geometridae develops regular outbreaks with decennal frequencies. This paper presents a historic of infestations on last 52 years (1960-2012) and analyzes the evolution trend of loopermoth populations in the last outbreak in Romania (2007-2010), depending by site conditions and forest stand characteristics. The mixed forests with common oak (Quercus robur) and sessile oak (Quercus petrea) as dominant species, placed on elevated plain, or on upper sides of the southern slopes, with ages over 80 years, shown favorable for developing ample outbreaks, with exponential growth rate of loopermoth populations. 1. INTRODUCTION In deciduous forests of Romania, according with statistics from last six decades, the defoliating insects (Lepidoptera) have had the highest percentage, in terms of infested areas. From the group of defoliating insects, the infestations produced by Tortrix viridana L. represents 44%, Geometridae sp. 31,7%, Lymntria dispar L. 21.6% and other species 2,7% (Malacosoma neustria L., Euproctis chrysorrhoea L, Thaumaetopoea processionea L. etc). Between the species of Geometridae, the most frequent, in orders of its importance, are Operophtera brumata L, Erannis defoliaria Cl., Erannis aurantiaria Hb., Erannis leucophaearia Schiff., Erannis marginaria F., Phigalia pedaria F., Alsophila aescularia Schiff. -
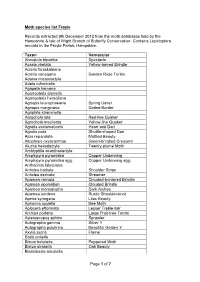
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

2020 Forest Action Plan
Alabama’s Forest Road Map 2020 The Forest Action Plan of the Alabama Forestry Commission Est. 1924 Welcome from the state forester Rick Oates, State Forester t is interesting how time modifies your perspective. Ten years ago, while working for the Alabama Forestry Association, I was asked to provide feedback in the development of the 2010 Alabama Forest Action Plan, Forests at the Crossroads. At the time I did not fully understand the importance of the Forest Action Plan to our state’s forest resources. IFast forward ten years and I am now the State Forester of Alabama, with a much better understanding of what this doc- ument means to the state. I now have the responsibility of updating this important plan. As such, it is with pride that I offer the 2020 Alabama Forest Action Plan, Alabama’s Forest Roadmap as a guide for all forestry stakeholders to reference over the next decade. This guide will serve as a tool to help our state better understand and manage this amazing resource. Alabama is blessed with abundant forest resources – 23.1 million acres - which cover more than two-thirds of the state. These forests improve water and air quality, provide wildlife habitat, support a growing forest industry and help provide jobs across the state. Without these forests Alabama would be a very different place. As such, we want to see forests remain as working forests in order to continue to accrue these important benefits. That is not to say there are not challenges associ- ated with our forest resource, but the assessment and strategies discussed in this document will be instrumental in raising awareness, implementing solutions and taking a step towards achieving this goal. -

Impact of Alien Insect Pests on Sardinian Landscape and Culture
Biodiversity Journal , 2012, 3 (4): 297-310 Impact of alien insect pests on Sardinian landscape and culture Roberto A. Pantaleoni 1, 2,* , Carlo Cesaroni 1, C. Simone Cossu 1, Salvatore Deliperi 2, Leonarda Fadda 1, Xenia Fois 1, Andrea Lentini 2, Achille Loi 2, Laura Loru 1, Alessandro Molinu 1, M. Tiziana Nuvoli 2, Wilson Ramassini 2, Antonio Sassu 1, Giuseppe Serra 1, Marcello Verdinelli 1 1Istituto per lo Studio degli Ecosistemi, Consiglio Nazionale delle Ricerche (ISE-CNR), traversa la Crucca 3, Regione Baldinca, 07100 Li Punti SS, Italy; e-mail: [email protected] 2Sezione di Patologia Vegetale ed Entomologia, Dipartimento di Agraria, Università degli Studi di Sassari, via Enrico De Nicola, 07100 Sassari SS, Italy; e-mail: [email protected] *Corresponding author ABSTRACT Geologically Sardinia is a raft which, for just under thirty million years, has been crossing the western Mediterranean, swaying like a pendulum from the Iberian to the Italian Peninsula. An island so large and distant from the other lands, except for its “sister” Corsica, has inevitably developed an autochthonous flora and fauna over such a long period of time. Organisms from other Mediterranean regions have added to this original contingent. These new arrivals were not randomly distributed over time but grouped into at least three great waves. The oldest two correspond with the Messinian salinity crisis about 7 million years ago and with the ice age, when, in both periods, Sardinia was linked to or near other lands due to a fall in sea level. The third, still in progress, is linked to human activity.