Predicting Forest Insect Disturbance Under Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementmaterial S2.Pdf
Mitt. Münch. Ent. Ges. 106 Suppl. S2 1-10 München, 15.02.2016 Systematische, revidierte und kommentierte Checkliste der Schmetterlinge Bayerns (Insecta: Lepidoptera) Alfred HASLBERGER & Andreas H. SEGERER Supplementmaterial S2 Zusammenstellung der in vorliegender Arbeit publizierten regionalen Neu- und Wiederfunde. S2.1 Neufunde für die Bayerischen Alpen und/oder das Alpenvorland (AVA) Nr. FauEu Überfamilie Familie Art 0016 431725 Eriocranioidea Eriocraniidae Dyseriocrania subpurpurella (HAWORTH, 1828) 0026 431739 Eriocranioidea Eriocraniidae Eriocrania semipurpurella (STEPHENS, 1835) 0058 431808 Nepticuloidea Nepticulidae Stigmella aceris (FREY, 1857) 0080 431900 Nepticuloidea Nepticulidae Stigmella myrtillella (STAINTON, 1857) 0089 431932 Nepticuloidea Nepticulidae Stigmella splendidissimella (HERRICH-SCHÄFFER, 1855) 0125 432021 Nepticuloidea Nepticulidae Ectoedemia decentella (HERRICH-SCHÄFFER, 1855) 0133 432060 Nepticuloidea Nepticulidae Ectoedemia hannoverella (GLITZ, 1872) 0158 432282 Adeloidea Heliozelidae Heliozela resplendella (STAINTON, 1851) 0182 432335 Adeloidea Adelidae Adela cuprella (DENIS & SCHIFFERMÜLLER, 1775) 0202 432387 Adeloidea Incurvariidae Incurvaria pectinea HAWORTH, 1828 0230 432437 Tischerioidea Tischeriidae Coptotriche marginea (HAWORTH, 1828) 0331 433122 Tineoidea Tineidae Nemapogon granella (LINNAEUS, 1758) 0355 432916 Tineoidea Tineidae Monopis weaverella (SCOTT, 1858) 0371 433010 Tineoidea Tineidae Tinea columbariella WOCKE, 1877 0373 433015 Tineoidea Tineidae Tinea trinotella THUNBERG, 1794 0394 433489 -

Motyle (Lepidoptera) Parku Krajobrazowego Cysterskie Kompozycje Krajobrazowe Rud Wielkich
ROCZNIK MUZEUM GÓRNOŚLĄSKIEGO W BYTOMIU PRZYRODA Vol. 26 (online 001): 1–40 ISSN 0068-466X, eISSN 2451-0467 (online) Bytom, 10.04.2020 Jacek Maroń1, Adam Larysz2 Motyle (Lepidoptera) Parku Krajobrazowego Cysterskie Kompozycje Krajobrazowe Rud Wielkich http://doi.org/10.5281/zenodo.3747209 1 ul. Kuglera 9, 44-207 Rybnik, Polska, e-mail: [email protected] 2 Dział Przyrody, Muzeum Górnośląskie w Bytomu, pl. Jana III Sobieskiego 2, 41-902 Bytom, Polska, e-mail: [email protected] Abstract: The Butterflies and Moths (Lepidoptera) of the Cysterskie Kompozycje Krajobrazowe Rud Wielkich Landscape Park. The paper presents the research on Lepidoptera in Cysterskie Kompozycje Krajobrazowe Rud Wielkich Landscape Park between 1982 and 2019. The material was collected at nine selected sites, mainly in various forest environments. Overall, 1162 species in 66 families were recorded and listed, including 26 species new to the Province of Silesia. Key words: Lepidoptera, list of species, Cysterskie Kompozycje Krajobrazowe Rud Wielkich Landscape Park, biodiversity. WSTĘP Park Krajobrazowy Cysterskie Kompozycje Krajobrazowe Rud Wielkich (PK CKKRW) położony jest w południowo-zachodniej części województwa śląskiego i zajmuje wschodnią część Kotliny Raciborskiej oraz północne fragmenty Płaskowyżu Rybnickiego. Powstał na mocy Rozporządzenia Wojewody Katowickiego Nr 181/93 z dnia 23 listopada 1993 roku. Powierzchnia Parku wynosi 493,87 km², a strefa ochronna to obszar 140,10 km². Dominują tu drzewostany sosnowe, rosnące na siedliskach borowych, wykształconych na glebach bielicowych, a także wprowadzone sztucznie na siedliska żyznych lasów liściastych. Najbogatszym przyrodniczo terenem w obrębie Parku jest kompleks leśno-stawowy Łężczok w okolicach Raciborza, będący jedynym rezerwatem w granicach Parku. MATERIAŁ I METODY PROWADZENIA BADAŃ Stopień poznania entomofauny PK CKKRW jest niezadawalający, a dane dotyczące występowania poszczególnych gatunków są fragmentaryczne i rozproszone. -
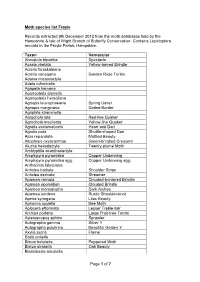
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Bosco Palazzi
SHILAP Revista de Lepidopterología ISSN: 0300-5267 ISSN: 2340-4078 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Bella, S; Parenzan, P.; Russo, P. Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 46, no. 182, 2018, April-June, pp. 315-345 Sociedad Hispano-Luso-Americana de Lepidopterología España Available in: https://www.redalyc.org/articulo.oa?id=45559600012 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative SHILAP Revta. lepid., 46 (182) junio 2018: 315-345 eISSN: 2340-4078 ISSN: 0300-5267 Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) S. Bella, P. Parenzan & P. Russo Abstract This study summarises the known records of the Macrolepidoptera species of the “Bosco Palazzi” area near the municipality of Putignano (Apulia region) in the Murgia mountains in southern Italy. The list of species is based on historical bibliographic data along with new material collected by other entomologists in the last few decades. A total of 207 species belonging to the families Cossidae (3 species), Drepanidae (4 species), Lasiocampidae (7 species), Limacodidae (1 species), Saturniidae (2 species), Sphingidae (5 species), Brahmaeidae (1 species), Geometridae (55 species), Notodontidae (5 species), Nolidae (3 species), Euteliidae (1 species), Noctuidae (96 species), and Erebidae (24 species) were identified. -

Redalyc.Temperature and Foliage Quality Affect Performance of The
Revista Chilena de Historia Natural ISSN: 0716-078X [email protected] Sociedad de Biología de Chile Chile PARITSIS, JUAN; VEBLEN, THOMAS T. Temperature and foliage quality affect performance of the outbreak defoliator Ormiscodes amphimone (F.) (Lepidoptera: Saturniidae) in northwestern Patagonia, Argentina Revista Chilena de Historia Natural, vol. 83, núm. 4, 2010, pp. 593-603 Sociedad de Biología de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=369944296012 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative TEMPERATURE AND DIET EFFECTS ON A DEFOLIATOR 593 REVISTA CHILENA DE HISTORIA NATURAL Revista Chilena de Historia Natural 83: 593-603, 2010 © Sociedad de Biología de Chile RESEARCH ARTICLE Temperature and foliage quality affect performance of the outbreak defoliator Ormiscodes amphimone (F.) (Lepidoptera: Saturniidae) in northwestern Patagonia, Argentina La temperatura y la calidad del follaje afectan el desempeño del defoliador epidémico Ormiscodes amphimone (F.) (Lepidoptera: Saturniidae) en el noroeste de la Patagonia argentina JUAN PARITSIS1,* & THOMAS T. VEBLEN1 1 Biogeography Lab, Department of Geography, University of Colorado, Boulder, Campus Box 260, CO 80309-0260, USA *Corresponding author: [email protected] ABSTRACT In the temperate forests of Chile and Argentina the phytophagous moth Ormiscodes amphimone (F.) causes severe defoliation on the southern beech tree Nothofagus pumilio (Poepp. & Endl.) Krasser. The recent increase in defoliation frequency in some areas appears to be influenced by a warmer climate. To evaluate the effects of temperature and the spatial heterogeneity of foliage quality on the performance and relative consumption rate of O. -

Vorarlbergs (Österreich) - Erkenntnisse Und Rückschlüsse : Supplement 2 1-29 Huemer, P
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Inatura Forschung online Jahr/Year: 2015 Band/Volume: 15_Supp2 Autor(en)/Author(s): Huemer Peter, Hebert Paul D. N. Artikel/Article: DNA-Barcoding der Schmetterlinge (Lepidoptera) Vorarlbergs (Österreich) - Erkenntnisse und Rückschlüsse : Supplement 2 1-29 Huemer, P. & Hebert, P.D.N. (2015): «DNA-Barcoding der Schmetterlinge (Lepidoptera) Vorarlbergs (Österreich) - Erkenntnisse und Rückschlüsse». inatura – Forschung online, Nr. 15, Suppl. 2: 29 S. DNA-Barcoding der Schmetterlinge (Lepidoptera) Nr. 15 - 2015 Vorarlbergs (Österreich) - Erkenntnisse und Suppl. 2 Rückschlüsse : Supplement 2 Peter Huemer1 & Paul D. N. Hebert2 1 Dr. Peter Huemer, Naturwissenschaftliche Sammlungen, Tiroler Landesmuseen Betriebsges.m.b.H., Feldstr. 11a, A-6020 Innsbruck. E-Mail: [email protected] 2 Prof. Dr. Paul D. N. Hebert, Biodiversity Institute of Ontario, University of Guelph, Guelph, ON, N1G 2W1, Canada. Anhangstabelle 2 Minimale genetische Distanzen zum jeweils Nächsten Nachbarn für 1497 Schmetterlingsarten aus Vorarlberg Familie Art Nächster Nachbar (NN) Distanz NN Adelidae Adela albicinctella Adela cuprella 2,51 Adelidae Adela cuprella Adela albicinctella 2,51 Adelidae Adela reaumurella Adela cuprella 7,15 Adelidae Cauchas rufimitrella Adela reaumurella 9,91 Adelidae Nematopogon metaxella Nematopogon schwarziellus 7,78 Adelidae Nematopogon pilella Nematopogon robertella 8,1 Adelidae Nematopogon -

Exposure of Noctuid and Geometrid Development Stages in Oak Forests
Glavendekić & Mihajlović: Exposure of noctuid and geometrid development stages in oak forests EXPOSURE OF NOCTUID AND GEOMETRID DEVELOPMENT STAGES IN OAK FORESTS Milka M. Glavendekić, Ljubodrag S. Mihajlović Faculty of Forestry University of Belgrade, Kneza Višeslava 1, 11030 Belgrade, Serbia (e-mail: [email protected]) Abstract The research was carried out in the period 1985-2005 in oak forests in Serbia. During the research of winter moths in oak forests of Serbia we recorded 9 outbreaking winter moth species: Colotois pennaria L., Agriopis aurantiaria Hbn., Erannis defoliaria Cl., Alsophila aescularia D.& Sch., A. aceraria D.& Sch., Operophtera brumata L., Apocheima pilosaria D.& Sch., Agriopis leucophaearia D.& Sch., A. marginaria F. The most significant noctuids are Orthosia spp. There are 38 parasitoids of the winter moths recorded and they fall into the families Braconidae, Ichneumonidae, Eulophidae, Torymidae, Trichogrammatidae, Scelionidae and Tachinidae. Egg parasitoids were recorded at 3 localities: Brankovac, Mala Moštanica and wide area of National Park Djerdap. Hyperparasitoids of winter moths in Serbia have also been studied. We recorded altogether 12 species of winter moth hyperparasitoids. The method of exposure was applied in egg and larva stages. While total parasitism in the natural population varies between 12.50-28.57 %, in the exposed conditions there were between 27.31-43.75 % parasitized caterpillars were recorded. The method of host exposure could be suitable for the local increase of abundance of parasitoids of winter moth eggs, larvae and pupae. Keywords: Defoliators, oak, Geometridae, Noctuidae 1. Introduction In Serbia forests cover about 2,313,000 ha, or 26.2 % of the whole territory. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Second Generation Sequencing and Morphological Faecal Analysis
Hope et al. Frontiers in Zoology 2014, 11:39 http://www.frontiersinzoology.com/content/11/1/39 RESEARCH Open Access Second generation sequencing and morphological faecal analysis reveal unexpected foraging behaviour by Myotis nattereri (Chiroptera, Vespertilionidae) in winter Paul R Hope1,2*†, Kristine Bohmann1,3†, M Thomas P Gilbert3, Marie Lisandra Zepeda-Mendoza3, Orly Razgour1 and Gareth Jones1 Abstract Background: Temperate winters produce extreme energetic challenges for small insectivorous mammals. Some bat species inhabiting locations with mild temperate winters forage during brief inter-torpor normothermic periods of activity. However, the winter diet of bats in mild temperate locations is studied infrequently. Although microscopic analyses of faeces have traditionally been used to characterise bat diet, recently the coupling of PCR with second generation sequencing has offered the potential to further advance our understanding of animal dietary composition and foraging behaviour by allowing identification of a much greater proportion of prey items often with increased taxonomic resolution. We used morphological analysis and Illumina-based second generation sequencing to study the winter diet of Natterer’sbat(Myotis nattereri) and compared the results obtained from these two approaches. For the first time, we demonstrate the applicability of the Illumina MiSeq platform as a data generation source for bat dietary analyses. Results: Faecal pellets collected from a hibernation site in southern England during two winters (December-March 2009–10 and 2010–11), indicated that M. nattereri forages throughout winter at least in a location with a mild winter climate. Through morphological analysis, arthropod fragments from seven taxonomic orders were identified. A high proportion of these was non-volant (67.9% of faecal pellets) and unexpectedly included many lepidopteran larvae. -

Climate Change Effects on Eruptive Forest Insects: a R Eview and Synthesis of Empirical Evidence
CLIMATE CHANGE EFFECTS ON ERUPTIVE FOREST INSECTS: A REVIEW AND SYNTHESIS OF EMPIRICAL EVIDENCE FRST 498 AMBERLY MARCINIAK ABSTRACT Global climate change is affecting ecosystems through warming temperatures, changing precipitation, and increasing climatic variability. One of the major impacts is the alteration of forest disturbance regimes, including forest insect outbreaks that cause landscape-scale tree mortality and significantly affect the composition, function, and socioeconomic value of forests. Many studies have been conducted and models created to predict how future climate change may affect forest insects, but it may also be useful to determine how insects have already responded in order to detect where knowledge may be lacking and which species may be of most concern in future forest management. For this thesis, research papers providing empirical evidence to show a definitive climate change effect on an eruptive forest insect were identified and reviewed. The selected papers were then synthesized into a predictive framework for the likely responses of specific forest insect groups or species to changes in temperature or precipitation. Bark beetles and defoliators were the two functional groups for which evidence was found. All evidence pointed to bark beetle species responding positively to warming temperatures and decreasing precipitation through range expansion and increases in outbreak extent and severity. Evidence was less straightforward for defoliators, as some species, but not all, showed a negative response to warmer -

DE NEDERLANDSE NAMEN. Van De Kleine Vlinders
DE NEDERLANDSE NAMEN van de kleine vlinders (microlepidoptera) in Nederland en België Nederlandse namen van de microlepidoptera Eindredactie: T.S.T. Muus & J.H.H. Zwier Samenstelling: Namens de Nederlandse Entomologische Vereniging, C. Gielis F. Groenen M.C.M. Jansen K.J. Huisman J.C. Koster T.S.T. Muus E.J. van Nieukerken M.J. van der Straten J.H.H. Zwier [email protected] 30 oktober 2009 Voorzijde: Thisanotia chrysonuchella, door J.C. Koster. Fotografie in deze uitgave door T.S.T. Muus. 2 Nederlandse namen van de microlepidoptera Inhoud 1. Inleiding.................................................................................................................................................... 4 2. Richtlijnen en methodiek ......................................................................................................................... 5 3. Bronnen.................................................................................................................................................... 9 4. Naamlijst van de Nederlandse soorten.................................................................................................. 10 Micropterigidae – oermotten ........................................................................................................... 10 Eriocraniidae – purpermotten........................................................................................................... 10 Opostegidae – oogklepmotten ........................................................................................................