Molecular Phylogenetics and Evolution 162 (2021) 107198
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A New Macrolepidopteran Moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican Amber
ZooKeys 965: 73–84 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.965.54461 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber Weiting Zhang1,2, Chungkun Shih3,4, YuHong Shih5, Dong Ren3 1 Hebei GEO University, 136 Huaiandonglu, Shijiazhuang 050031, China 2 State Key Laboratory of Pal- aeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, CAS, Nanjing 210008, China 3 College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, 105 Xisan- huanbeilu, Haidian District, Beijing 100048, China 4 Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, USA 5 Laboratorio Dominicano De Ambar Y Gemas, Santo Domingo, Dominican Republic Corresponding author: Weiting Zhang ([email protected]) Academic editor: Gunnar Brehm | Received 19 May 2020 | Accepted 12 August 2020 | Published 3 September 2020 http://zoobank.org/05E273DB-B590-42D1-8234-864A787BE6A0 Citation: Zhang W, Shih C, Shih YH, Ren D (2020) A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber. ZooKeys 965: 73–84. https://doi.org/10.3897/zookeys.965.54461 Abstract A new genus and species of fossil moth, Miogeometrida chunjenshihi Zhang, Shih & Shih, gen. et sp. nov., assigned to Geometridae, is described from Miocene Dominican amber dating from 15–20 Mya. The new genus is characterized by the forewing without a fovea, R1 not anastomosing with Sc, no areole formed by veins R1 and Rs, R1 and Rs1 completely coincident, M2 arising midway between M1 and M3, anal veins 1A and 2A fused for their entire lengths; and the hind wing with Rs running close to Sc + R1 and M2 absent. -

Classical Biological Control of Arthropods in Australia
Classical Biological Contents Control of Arthropods Arthropod index in Australia General index List of targets D.F. Waterhouse D.P.A. Sands CSIRo Entomology Australian Centre for International Agricultural Research Canberra 2001 Back Forward Contents Arthropod index General index List of targets The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its primary mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has special competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MONOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR’s research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for International Agricultural Research, GPO Box 1571, Canberra ACT 2601, Australia Waterhouse, D.F. and Sands, D.P.A. 2001. Classical biological control of arthropods in Australia. ACIAR Monograph No. 77, 560 pages. ISBN 0 642 45709 3 (print) ISBN 0 642 45710 7 (electronic) Published in association with CSIRO Entomology (Canberra) and CSIRO Publishing (Melbourne) Scientific editing by Dr Mary Webb, Arawang Editorial, Canberra Design and typesetting by ClarusDesign, Canberra Printed by Brown Prior Anderson, Melbourne Cover: An ichneumonid parasitoid Megarhyssa nortoni ovipositing on a larva of sirex wood wasp, Sirex noctilio. Back Forward Contents Arthropod index General index Foreword List of targets WHEN THE CSIR Division of Economic Entomology, now Commonwealth Scientific and Industrial Research Organisation (CSIRO) Entomology, was established in 1928, classical biological control was given as one of its core activities. -

Iridopsis Socoromaensis Sp. N., a Geometrid Moth (Lepidoptera, Geometridae) from the Andes of Northern Chile
Biodiversity Data Journal 9: e61592 doi: 10.3897/BDJ.9.e61592 Taxonomic Paper Iridopsis socoromaensis sp. n., a geometrid moth (Lepidoptera, Geometridae) from the Andes of northern Chile Héctor A. Vargas ‡ ‡ Universidad Tarapacá, Arica, Chile Corresponding author: Héctor A. Vargas ([email protected]) Academic editor: Axel Hausmann Received: 02 Dec 2020 | Accepted: 26 Jan 2021 | Published: 28 Jan 2021 Citation: Vargas HA (2021) Iridopsis socoromaensis sp. n., a geometrid moth (Lepidoptera, Geometridae) from the Andes of northern Chile. Biodiversity Data Journal 9: e61592. https://doi.org/10.3897/BDJ.9.e61592 ZooBank: urn:lsid:zoobank.org:pub:3D37F554-E2DC-443C-B11A-8C7E32D88F4F Abstract Background Iridopsis Warren, 1894 (Lepidoptera: Geometridae: Ennominae: Boarmiini) is a New World moth genus mainly diversified in the Neotropical Region. It is represented in Chile by two described species, both from the Atacama Desert. New information Iridopsis socoromaensis sp. n. (Lepidoptera: Geometridae: Ennominae: Boarmiini) is described and illustrated from the western slopes of the Andes of northern Chile. Its larvae were found feeding on leaves of the Chilean endemic shrub Dalea pennellii (J.F. Macbr.) J.F. Macbr. var. chilensis Barneby (Fabaceae). Morphological differences of I. socoromaensis sp. n. with the two species of the genus previously known from Chile are discussed. A DNA barcode fragment of I. socoromaensis sp. n. showed 93.7-94.3% similarity with the Nearctic I. sanctissima (Barnes & McDunnough, 1917). However, the morphology of the genitalia suggests that these two species are distantly related. The © Vargas H. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

(Prout)(Geometridae
The LepidopterologicalSocietyLepidopterological Society of Japan eetva Z'ans. tepid. Soc. Jopan 59 (2): 171-185,March 2008 Notes onAlcis variegata (Moore),A. colorijlera (Prout) (Geometridae, Ennominae), and their allies from the Sunda Islands, with descriptions of two new species Rikio SATo 2-27-29, Shindori-nishi, Nishi-ku, Niigata, 950-2036 Japan Abstract Two species-groups ofAlcis from the Sunda Islands are revised. The A. variegata cum- plex: A, variegata (Moore), A. com,ariata (Prout), stat. & comb. nov,, A. hemiphanes (ProuO, A. cockaynei (Prout), A. Iuizi sp. nov. (Sumatra), A. paukstadti sp. nov, (Flores>, A. praevariegata (ProuO. Alcis taiwanovariegata nom. nov. is proposed for Boarmia suhochrearia Wileman & South, 1917, currently assigned to Alcis and preeccupied by Leech, IS97, The A. colorCfera com- plex: A. coloritlera (Prout), A. periphracta (Prout). Key words Geumetridae, Ennominae, Atcis variegata, Alcis color(fera, taxonomic notes, new species, Sunda Islands. In my previous paper (Sato, 2005), two species groups of the genus Atcis Cunis from Southeast Asia, the pammicra and maculata cQmplexes, were revised. In this paper, I will give a taxonomic account of two further species groups of Alcis from the Sunda Islands (Borneo, Sumatra, Java, Flores) in Southeast Asia, with descriptions of two new species, A, "variegata variegata andA. color(feva, and species allied to them will be named here the L`colorijleia complex" and complex" without strict definition, for the sake of convenience, No species belonging to the two complexes have been found from Sulawesi. For precise identification of the taxonomically more confusing species, I examined all available type specimens and their genitalia at the Natura] History Museum, London, UK, when I visited there in 2002. -

Poplars and Willows: Trees for Society and the Environment / Edited by J.G
Poplars and Willows Trees for Society and the Environment This volume is respectfully dedicated to the memory of Victor Steenackers. Vic, as he was known to his friends, was born in Weelde, Belgium, in 1928. His life was devoted to his family – his wife, Joanna, his 9 children and his 23 grandchildren. His career was devoted to the study and improve- ment of poplars, particularly through poplar breeding. As Director of the Poplar Research Institute at Geraardsbergen, Belgium, he pursued a lifelong scientific interest in poplars and encouraged others to share his passion. As a member of the Executive Committee of the International Poplar Commission for many years, and as its Chair from 1988 to 2000, he was a much-loved mentor and powerful advocate, spreading scientific knowledge of poplars and willows worldwide throughout the many member countries of the IPC. This book is in many ways part of the legacy of Vic Steenackers, many of its contributing authors having learned from his guidance and dedication. Vic Steenackers passed away at Aalst, Belgium, in August 2010, but his work is carried on by others, including mem- bers of his family. Poplars and Willows Trees for Society and the Environment Edited by J.G. Isebrands Environmental Forestry Consultants LLC, New London, Wisconsin, USA and J. Richardson Poplar Council of Canada, Ottawa, Ontario, Canada Published by The Food and Agriculture Organization of the United Nations and CABI CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © FAO, 2014 FAO encourages the use, reproduction and dissemination of material in this information product. -

Conservation Assessment for the Kansan Spikerush Leafhopper (Dorydiella Kansana Beamer)
Conservation Assessment For The Kansan spikerush leafhopper (Dorydiella kansana Beamer) USDA Forest Service, Eastern Region January 11, 2005 James Bess OTIS Enterprises 13501 south 750 west Wanatah, Indiana 46390 This document is undergoing peer review, comments welcome This Conservation Assessment was prepared to compile the published and unpublished information on the subject taxon or community; or this document was prepared by another organization and provides information to serve as a Conservation Assessment for the Eastern Region of the Forest Service. It does not represent a management decision by the U.S. Forest Service. Though the best scientific information available was used and subject experts were consulted in preparation of this document, it is expected that new information will arise. In the spirit of continuous learning and adaptive management, if you have information that will assist in conserving the subject taxon, please contact the Eastern Region of the Forest Service - Threatened and Endangered Species Program at 310 Wisconsin Avenue, Suite 580 Milwaukee, Wisconsin 53203. TABLE OF CONTENTS EXECUTIVE SUMMARY ............................................................................................................ 1 ACKNOWLEDGEMENTS............................................................................................................ 1 NOMENCLATURE AND TAXONOMY ..................................................................................... 1 DESCRIPTION OF SPECIES....................................................................................................... -

Lepidoptera of a Raised Bog and Adjacent Forest in Lithuania
Eur. J. Entomol. 101: 63–67, 2004 ISSN 1210-5759 Lepidoptera of a raised bog and adjacent forest in Lithuania DALIUS DAPKUS Department of Zoology, Vilnius Pedagogical University, Studentų 39, LT–2004 Vilnius, Lithuania; e-mail: [email protected] Key words. Lepidoptera, tyrphobiontic and tyrphophilous species, communities, raised bog, wet forest, Lithuania Abstract. Studies on nocturnal Lepidoptera were carried out on the Laukėnai raised bog and the adjacent wet forest in 2001. Species composition and abundance were evaluated and compared. The species richness was much higher in the forest than at the bog. The core of each lepidopteran community was composed of 22 species with an abundance of higher than 1.0% of the total catch. Tyrpho- philous Hypenodes humidalis (22.0% of all individuals) and Nola aerugula (13.0%) were the dominant species in the raised bog community, while tyrphoneutral Pelosia muscerda (13.6%) and Eilema griseola (8.3%) were the most abundant species at the forest site. Five tyrphobiotic and nine tyrphophilous species made up 43.4% of the total catch on the bog, and three and seven species, respectively, at the forest site, where they made up 9.2% of all individuals. 59% of lepidopteran species recorded on the bog and 36% at the forest site were represented by less than five individuals. The species compositions of these communities showed a weak similarity. Habitat preferences of the tyrphobiontic and tyrphophilous species and dispersal of some of the species between the habi- tats are discussed. INTRODUCTION (1996). Ecological terminology is that of Mikkola & Spitzer (1983), Spitzer & Jaroš (1993), Spitzer (1994): tyrphobiontic The insect fauna of isolated raised bogs in Europe is species are species that are strongly associated with peat bogs, unique in having a considerable portion of relict boreal while tyrphophilous taxa are more abundant on bogs than in and subarctic species (Mikkola & Spitzer, 1983; Spitzer adjacent habitats. -
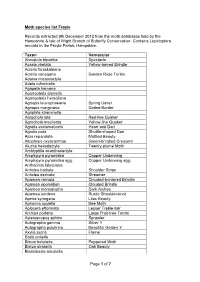
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Proceedings of the Tenth Forum Herbulot 2018. Integrative Taxonomy, a Multidisciplinary Approach to Answer Compli- Cated Taxonomic Questions
SPIXIANA 42 2 291-320 München, Dezember 2019 ISSN 0341-8391 Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer compli- cated taxonomic questions (Stuttgart, Germany, 11-16 June 2018) Axel Hausmann & Hossein Rajaei (eds) Hausmann, A. & Rajaei, H. (eds) 2019. Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions (Stuttgart, Germany, 11-16 June 2018). Spixiana 42 (2): 291- 320. The tenth International Congress of FORUM HERBULOT on “Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions” took place in the Staatliches Museum für Naturkunde Stuttgart (SMNS), from 11.- 16.06.2018, with 77 participants and 52 scientific presentations. The proceedings provide short information on the meeting and the abstracts of the oral presenta- tions. Axel Hausmann (corresponding author), SNSB – ZSM, Bavarian State Collection of Zoology, Münchhausenstr. 21, 81247 Munich, Germany; e-mail: [email protected] Short report and results Axel Hausmann & Hossein Rajaei Hausmann, A. & Rajaei, H. 2019. Short report and results. Pp. 291-292 in: Hausmann, A. & Rajaei, H. (eds). Proceedings of the tenth FORUM HERBULOT 2018. Integrative taxonomy, a multidisciplinary approach to answer complicated taxonomic questions (Stuttgart, Germany, 11-16 June 2018). Spixiana 42 (2). Axel Hausmann (corresponding author), SNSB – ZSM, Bavarian State Collection of Zoology, Münchhausenstr. 21, 81247 Mu- nich, Germany; e-mail: [email protected] The meeting was organized by an organization The conference started with a lecture on the ground- team of the ‘Staatliches Museum für Naturkunde breaking effects of “Willi Hennig and the synthesis of Stuttgart’ (SMNS). -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Moths of North Carolina - Early Draft 1
Geometridae Cleora projecta Projecta Gray Moth 40 n=0 30 High Mt. N 20 u • m 10 b e 0 • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 10 • • Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o 40 • • f n=0 • = Sighting or Collection 30 • Low Mt. High counts of: • in NC since 2001 F • = Not seen since 2001 l 20 37 - Brunswick - 1994-03-19 • i 10 24 - Brunswick - 2006-04-24 g Status Rank h 24 - Pender - 1995-04-17 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 40 40 t n=0 n=89 e 30 Pd 30 CP s 20 20 10 10 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Geometridae SUBFAMILY: Ennominae TRIBE: Boarmiini TAXONOMIC_COMMENTS: This genus occurs over much of the world but in North America there are only two species and both occur in North Carolina FIELD GUIDE DESCRIPTIONS: Covell (1984) ONLINE PHOTOS: TECHNICAL DESCRIPTION, ADULTS: Forbes (1948; as C. -

Bosco Palazzi
SHILAP Revista de Lepidopterología ISSN: 0300-5267 ISSN: 2340-4078 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Bella, S; Parenzan, P.; Russo, P. Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 46, no. 182, 2018, April-June, pp. 315-345 Sociedad Hispano-Luso-Americana de Lepidopterología España Available in: https://www.redalyc.org/articulo.oa?id=45559600012 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative SHILAP Revta. lepid., 46 (182) junio 2018: 315-345 eISSN: 2340-4078 ISSN: 0300-5267 Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) S. Bella, P. Parenzan & P. Russo Abstract This study summarises the known records of the Macrolepidoptera species of the “Bosco Palazzi” area near the municipality of Putignano (Apulia region) in the Murgia mountains in southern Italy. The list of species is based on historical bibliographic data along with new material collected by other entomologists in the last few decades. A total of 207 species belonging to the families Cossidae (3 species), Drepanidae (4 species), Lasiocampidae (7 species), Limacodidae (1 species), Saturniidae (2 species), Sphingidae (5 species), Brahmaeidae (1 species), Geometridae (55 species), Notodontidae (5 species), Nolidae (3 species), Euteliidae (1 species), Noctuidae (96 species), and Erebidae (24 species) were identified.