吉林省輝南長龍生化藥業股份有限公司 Annual Report 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

대외경제정책연구원-2014 KIEP Visiting Fellows Program.Hwp
2014 2014 KIEP KIEP Visiting Fellows Program KIEP Fellows Visiting Visiting Fellows Program Edited by JEONG Hyung-Gon Edited by JEONG Hyung-Gon 370 Sicheong-daero, Sejong-Si 339-705, Korea Tel: (8244) 414-1042 / Fax: (8244) 414-1043 URL: http://www.kiep.go.kr 2014 KIEP Visiting Fellows Program Edited by JEONG Hyung-Gon The Contents of the KIEP Visiting Fellow Program do not reflect or represent the official opinion of KIEP. The KIEP Visiting Fellows Program is published with the aim of promoting discussions among researchers, and to remember the outstanding achievements by the visiting fellows who came to KIEP. KOREA INSTITUTE FOR INTERNATIONAL ECONOMIC POLICY (KIEP) 370 Sicheong-daero, Sejong-Si 339-705, Korea Tel: (8244) 414-1042 Fax: (8244) 414-1043 URL: http://www.kiep.go.kr LEE Il Houng, President Published 2015 in Korea by KIEP ⓒ 2015 KIEP Acknowledgements In 2009, Korea Institute for International Economic Policy (KIEP) launched "Visiting Fellows Program (VFP)" with the view of advancing cross-border exchanges of knowledge, information, insights and expertise. Since its inception, the VFP has demonstrated that sharing thoughts and ideas through face-to-face contacts and dialogue works as a catalyst for enhancing mutual understanding among scholars and professionals with diverse background. By successfully implementing the VFP for the past 7 years, KIEP has been motivated to assume the role as a hub for international economic research in the region. As a host of the program, KIEP has many mandates. One of those tasks is to let more people know what has been accomplished through the program and how valuable it is. -

2.15 Jilin Province Jilin Province Jixin Group Co. Ltd., Affiliated to the Jilin Provincial Prison Administration Bureau, Has 22
2.15 Jilin Province Jilin Province Jixin Group Co. Ltd., affiliated to the Jilin Provincial Prison Administration Bureau, has 22 prison enterprises Legal representative of the prison company: Feng Gang, Chairman of Jilin Jixin Group Co., Ltd. His official positions in the prison system: Party Committee Member of Jilin Provincial Justice Department, Party Committee Secretary and Director of Jilin Provincial Prison Administration Bureau1 According to the “Notice on Issuing ‘Jilin Province People’s Government Institutional Reform Program’ from the General Office of the CCP Central Committee and the General Office of the State Council” (Ting Zi [2008] No. 25), the Jilin Provincial Prison Administration Bureau (Deputy-department level) was set up as a management agency under the Provincial Justice Department.2 Business areas: The company manages state-owned operating assets of the enterprises within province’s prison system; production, processing and sale of electromechanical equipment (excluding cars), chemical products, apparels, cement, construction materials; production and sale of agricultural and sideline products; labor processing No. Company Name of the Legal Person Legal Registered Business Scope Company Notes on the Prison Name Prison, to which and representative Capital Address the Company Shareholder(s) / Title Belongs 1 Jilin Jixin Jilin Provincial State-owned Feng Gang 70.67 The company manages state-owned 1000 Xinfa According to the “Notice on Issuing Group Co., Prison Asset Chairman of Jilin million operating assets of the -

Printmgr File
APPENDIX VII STATUTORY AND GENERAL INFORMATION A. FURTHER INFORMATION ABOUT THE BANK 1. Incorporation On December 15, 2008, upon the approval of the CBRC Jilin Bureau, the Bank was promoted and established as a joint stock commercial bank named “Jilin Jiutai Rural Commercial Bank Corporation Limited” ( ) by qualified natural person shareholders of the former Jiutai Rural Credit Cooperative ( ), newly introduced natural person shareholders and legal person shareholders. On December 16, 2008, the Bank was formally incorporated. The Bank’s current registered address is No. 504 Xinhua Main Street, Jiutai District, Changchun, Jilin province, the PRC. The Bank has established a place of business in Hong Kong at Room 3521, 35/F, Two Pacific Place, 88 Queensway, Hong Kong and registered as a non-Hong Kong company in Hong Kong on February 17, 2016 under Part XVI of the Companies Ordinance. The Bank appointed Wong Yat Tung as the Bank’s authorized representative for the acceptance of service of process and notices in Hong Kong. The address for service of process on the Bank in Hong Kong is at 18/F, Tesbury Centre, 28 Queen’s Road East, Wanchai, Hong Kong. As the Bank was established in the PRC, the Bank’s corporate structure and articles of association are subject to the relevant laws and regulations of the PRC. Certain aspects of the PRC laws and regulations and a summary of certain provisions of the Bank’s articles of association are set out in Appendices IV and V to this prospectus, respectively. The Bank is not an authorized institution within the meaning of the Banking Ordinance, and are not subject to the supervision of the HKMA, nor authorized to carry on banking and/or deposit-taking business in Hong Kong. -

The Comparison of Different Calculation Methods of Pollution Receiving Capacity for Jilin Province Huifa River
Nature Environment and Pollution Technology ISSN: 0972-6268 Vol. 15 No. 4 pp. 1169-1176 2016 An International Quarterly Scientific Journal Original Research Paper The Comparison of Different Calculation Methods of Pollution Receiving Capacity for Jilin Province Huifa River Yao Liwei and Men Baohui† Renewable Energy Institute, North China Electric Power University, Beijing-102206, China †Corresponding author: Men Baohui ABSTRACT Nat. Env. & Poll. Tech. Website: www.neptjournal.com Huifa River is the largest tributary of the Second Songhua River. Songhua River Basin is the concentrated area of Northeast Old Industrial Base, and it is also the distribution area of major cities, bearing Received: 19-12-2015 production task of national commodity grain. In recent years, with the rapid development of economy, Accepted: 28-01-2016 the deterioration of water quality is serious and the water environment problem is becoming more and Key Words: more outstanding, which have affected the sustainable development of the economic and social of Pollution receiving capacity Jilin province, so it is necessary to analyse and study the pollution receiving capacity of the river and Water quality model control the water pollution source to protect the water environment and strengthen water resources Sewage outfall protection. Based on one-dimensional water quality model, this paper use three kinds of different Huifa river generalization methods, such as midpoint generalization, uniform generalization and sewage outfall barycenter generalization, to calculate -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Multi-Destination Tourism in Greater Tumen Region
MULTI-DESTINATION TOURISM IN GREATER TUMEN REGION RESEARCH REPORT 2013 MULTI-DESTINATION TOURISM IN GREATER TUMEN REGION RESEARCH REPORT 2013 Greater Tumen Initiative Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH GTI Secretariat Regional Economic Cooperation and Integration in Asia (RCI) Tayuan Diplomatic Compound 1-1-142 Tayuan Diplomatic Office Bldg 1-14-1 No. 1 Xindong Lu, Chaoyang District No. 14 Liangmahe Nanlu, Chaoyang District Beijing, 100600, China Beijing, 100600, China www.tumenprogramme.org www.economicreform.cn Tel: +86-10-6532-5543 Tel: + 86-10-8532-5394 Fax: +86-10-6532-6465 Fax: +86-10-8532-5774 [email protected] [email protected] © 2013 by Greater Tumen Initiative The views expressed in this paper are those of the author and do not necessarily reflect the views and policies of the Greater Tumen Initiative (GTI) or members of its Consultative Commission and Tourism Board or the governments they represent. GTI does not guarantee the accuracy of the data included in this publication and accepts no responsibility for any consequence of their use. By making any designation of or reference to a particular territory or geographic area, or by using the term “country” in this document, GTI does not intend to make any judgments as to the legal or other status of any territory or area. “Multi-Destination Tourism in the Greater Tumen Region” is the report on respective research within the GTI Multi-Destination Tourism Project funded by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. The report was prepared by Mr. James MacGregor, sustainable tourism consultant (ecoplan.net). -

World Bank Document
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: ICR00003974 IMPLEMENTATION COMPLETION AND RESULTS REPORT Loan Number 7899-CN ON A LOAN Public Disclosure Authorized IN THE AMOUNT OF US$ 100 MILLION TO THE PEOPLE'S REPUBLIC OF CHINA FOR THE JILIN AGRICULTURAL PRODUCT SAFETY AND QUALITY ( P101716 ) December 18, 2017 Public Disclosure Authorized Agriculture Global Practice East Asia And Pacific Region Public Disclosure Authorized CURRENCY EQUIVALENTS (Exchange Rate Effective December 6, 2017) Currency Unit = Renminbi (RMB) RMB 6.61 = US$1 FISCAL YEAR January 1 – December 31 Regional Vice President: Victoria Kwakwa, EAPVP Country Director: Bert Hofman, EACCF Senior Global Practice Director: Juergen Voegle, GFADR Practice Manager: Nathan M. Belete, GFA02 Task Team Leader(s): Carolina V. Figueroa-Geron, GFA02 ICR Main Contributor: Xueming Liu, FAO/CP ABBREVIATIONS AND ACRONYMS CNAS China National Accreditation Service for Conformity Assessment CPS Country Partnership Strategy EMP Environmental Management Plan FAO Food & Agriculture Organization GAP Good Agricultural Practices IPM Integrated Pest Management ISO International Organization for Standardization M&E Monitoring and Evaluation MIS Management Information System PDO/PDOs Project Development Objective/s POCAD Provincial Office for Comprehensive Agricultural Development PIU Project Implementing Unit PPMO Provincial Project Management Office PRC Peoples’ Republic of China TABLE OF CONTENTS DATA SHEET .......................................................................................................................... -

External Monitoring Report of Resettlement for ADB Financed
Social Monitoring Report Project Number: 40665 October 2011 PRC: Songhua River Basin Water Pollution Control and Management Project (Jilin Component) Prepared by the Project Management Office of Jilin Provincial Government with the assistance of Northeast Project Management Co. Ltd. For: Jilin Provincial Government Dehui City Tianyi Water Co. Ltd.; Gongzhuling City Urban State-Owned Assets Operations Co. Ltd. Fusong County Lantianbishui Infrastructure Development Co. Ltd.; Fuyu County Wastewater Treatment Co. Ltd.; Jingyu County Yutong Municipal Engineering Co. Ltd.; Liuhe County Bishui Wastewater Treatment Co. Ltd.; Tonghua County Water Supply Co.; Yushu Tianyi Water Co. Ltd.; Da’an City Xingcheng Urban Infrastructure Development and Construction Co. Ltd. Huadian City Infrastructure Development Construction Investment Co. Ltd.; Huinan County Chaoyang Township Huashu Domestic SolidWaste Treatment Service Co. Ltd.; Jiaohe City Jiemei Solid Waste Co.; Jingyu County Huanyu Domestic Solid Waste Management Co. Ltd,; Liuhe County Luyuan Domestic Solid Waste Management and Service Co. Ltd.; Meihekou City Jiecheng Domestic Solid Waste Collection, Transportation and Treatment Co. Ltd.; Tongyu County Hecheng Domestic Solid Waste Sanitary Landfill; Changbaishan Development and Construction Group Co. Ltd. This report has been submitted to ADB by the Project Management Office of Hefei Municipal Government and is made publicly available in accordance with ADB’s public communications policy (2005). It does not necessarily reflect the views of ADB. ADB -

Changbai Wood-Rotting Fungi 5. Study on Polyporus Mongolicus and P. Tubaeformis
Ann. Bot. Fennici 33: 153–163 ISSN 0003-3847 Helsinki 18 June 1996 © Finnish Zoological and Botanical Publishing Board 1996 Changbai wood-rotting fungi 5. Study on Polyporus mongolicus and P. tubaeformis Yu-Cheng Dai Dai, Y.-C., Department of Ecology and Systematics, and Botanical Museum, P.O. Box 47, FIN-00014 University of Helsinki, Finland Received 2 January 1996, accepted 19 March 1996 Polyporus mongolicus (Pilát) Y. C. Dai, earlier treated as a variety of P. arcularius Batsch: Fr. by Pilát, is erected as an independent species. It is separated from the other pale-stiped Polyporus species (the Polyporellus P. Karst. group) in having duplex con- text, and fairly big and freely arranged pores; it has both simple-septate and clamped hyphae in the upper hirsute layer of the cap. Its affinities with the other species in the Polyporellus group are given. Another polypore species, growing on wood of gymno- sperms in NE Asia, is identified as P. tubaeformis (P. Karst.) Ryvarden & Gilb. It resembles P. melanopus (Pers.) Fr., but differs by having narrower generative hyphae, tightly interwoven tramal hyphae, thick-walled upper surface hyphae making up a pali- sade, and by bearing cystidioles. The differences between it and the other taxa in the black-stiped group (the Melanopus complex) are discussed. Polyporus hemicapnodes Berk & Broome, a predominantly tropical species, has been found in the Far East of Russia, and was now collected in N China (new to China). It is characterized by having small and slender basidiocarps, a black stipe, pale luteous upper surface, strongly de- current pores and subellipsoid spores. -

State-Run Industrial Enterprises in Fengtian, 1920-1931
State Building, Capitalism, and Development: State-Run Industrial Enterprises in Fengtian, 1920-1931 A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Yu Jiang IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Liping Wang, Ann Waltner October 2010 © Yu Jiang, 2010 Acknowledgements I am grateful that Department of History at University of Minnesota took a risk with me, somebody with no background in history. I also thank the department for providing a one-semester fellowship for my dissertation research. I am deeply indebted to Minnesota Population Center for hosting me in its IT team for many years – it allowed me, a former software engineer, to keep abreast of the latest developments in computer technology while indulging in historical studies. Thanks are especially due to Steve Ruggles (MPC director) and Pete Clark (IT director) for giving me this great opportunity. The source materials for this dissertation mostly come from Liaoning Provincial Archives, Liaoning Provincial Library, and Meihekou Archives. I thank these institutions for their help. Liaoning Shekeyuan offered both warm reception and administrative help right after I arrived in Shenyang. My archival research in Shenyang also benefited greatly from Chris Isett’s help. Su Chen from East Asian Library at University of Minnesota has been helpful numerous times in locating important documents. My committee members, Mark Anderson, Ted Farmer, M.J. Maynes, Ann Waltner, and Liping Wang provided valuable comments on the draft. M.J. and Ann’s thoughtful comments on a paper based on my dissertation helped improve my work. -

PRC: Jilin Component, Songhua River Basin Water Pollution Control And
Environmental Monitoring Report Project Number: 40665 September 2012 PRC: Songhua River Basin Water Pollution Control and Management Project, Jilin Component (ADB Loan No: 2487-PRC) Prepared by Jilin PMO with the Assistance of Easen International Co., Ltd. for the Jilin Provincial Government and the Asian Development Bank. This report has been submitted to ADB by the Project Management Office of Jilin Province and is made publicly available in accordance with ADB’s public communications policy (2005). It does not necessarily reflectThe views the viewsexpressed of ADB. herein Your are attention those ofis thedirected consul totant the and―Terms do not of Use‖necessarily section representof this website. those of ADB’s members, Board of Directors, Management, or staff, and may be preliminary in nature. TABLE OF CONTENT I. INTRODUCTION 2 A. Report Purpose and Rationale 2 B. Project Objective and Components 2 C. Project Implementation Progress 2 II. INSTITUTIONAL SETUP AND RESPONSIBILITIES FOR EMP IMPLEMENTATION AND SUPERVISION 5 A. Institutional responsibilities for environmental management 6 B. Incorporation of Environmental Requirements into Project Contractual Arrangements 6 III. COMPLIANCE WITH ENVIRONMENT RELATED PROJECT COVENANTS 7 Status of Compliance 7 IV. ENVIRONMENTAL MITIGATIONS AND COMPENSATION MEASURES IMPLEMENTED IN THE REPORTING PERIOD 9 V. SUMMARY OF ENVIRONMENTAL MONITORING 12 A. Monitoring plan and responsibilities 12 B. Environmental Audit upon Project Completion 13 C. Environmental quality targets, sampling and analytical methods 15 D. Monitoring Results 21 E. Assessment 30 VI. PUBLIC CONSULTATION 31 VII. INSTITUTIONAL STRENGTHENING AND TRAINING 33 VIII. KEY ENVIRONMENTAL ISSUES 36 IX. CONCLUSION 36 A. Overall Progress of Implementation of Environmental Management Measures 36 B. -
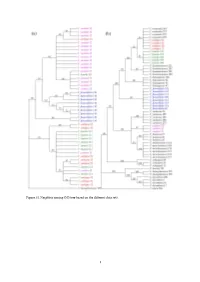
Neighbor Joining (NJ) Tree Based on the Different Data Sets
Figure S1: Neighbor joining (NJ) tree based on the different data sets. 1 Figure S2: Species identification efficiency based on “Best Match”. 2 Figure S3: Graph of ABGD web results using K80 Kimura measure of distance. Table S1: Collection details and NCBI accession numbers of samples used in this study. Locality of psbA- Species Longitude, Latitude and altitude matK rbcL trnG Collection trnH Corydalis ambigua Ji'an City, China E126°20′13.3″N41°22′42.8″H602.1m MF573564 MF573619 MF573674 MF573729 321 Corydalis ambigua 52 Ji'an City, China E126°20′14.2″N41°22′41.3″H614m MF573565 MF573620 MF573675 MF573730 Tonghua City, Corydalis ambigua 72 E126°01′3.7″N41°43′15.9″H588m MF573566 MF573621 MF573676 MF573731 China Linjiang City, Corydalis ambigua 92 E126°45′34.5″N41°54′21.8″H656m MF573567 MF573622 MF573677 MF573732 China 3 Baishan City, Corydalis buschii 322 E127°28′2.06″N42°09′34.45″ H753m MG748615 MG748617 MG748619 MG748621 China Suzhou City, Corydalis caudata 292 E116°52′30.75″N34°10′01.59″H78m MF573568 MF573623 MF573678 MF573733 China Luoyang City, Corydalis caudata 32 E111°50′15.81″N33°41′26.87″H1463m MF573569 MF573624 MF573679 MF573734 China Luoyang City, Corydalis caudata 35 E111°50′15.85″N33°41′25.18″H1490m MF573570 MF573625 MF573680 MF573735 China Corydalis decumbens Tongling City, E117°56′57.86″N30°54′07.75″H186m MF573571 MF573626 MF573681 MF573736 1 China Corydalis decumbens Chuzhou City, E117°33′36.09″N32°52′48.87″H59m MF573572 MF573627 MF573682 MF573737 16 China Corydalis decumbens Ma'anshan City, E118°34′53.74″N31°35′42.18″H130m