Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cocaine Intoxication and Hypertension
THE EMCREG-INTERNATIONAL CONSENSUS PANEL RECOMMENDATIONS Cocaine Intoxication and Hypertension Judd E. Hollander, MD From the Department of Emergency Medicine, University of Pennsylvania, Philadelphia, PA. 0196-0644/$-see front matter Copyright © 2008 by the American College of Emergency Physicians. doi:10.1016/j.annemergmed.2007.11.008 [Ann Emerg Med. 2008;51:S18-S20.] with cocaine intoxication is analogous to that of the patient with hypertension: the treatment should be geared toward the Cocaine toxicity has been reported in virtually all organ patient’s presenting complaint. systems. Many of the adverse effects of cocaine are similar to When the medical history is clear and symptoms are mild, adverse events that can result from either acute hypertensive laboratory evaluation is usually unnecessary. In contrast, if the crisis or chronic effects of hypertension. Recognizing when the patient has severe toxicity, evaluation should be geared toward specific disease requires treatment separate from cocaine toxicity the presenting complaint. Laboratory evaluation may include a is paramount to the treatment of patients with cocaine CBC count; determination of electrolyte, glucose, blood urea intoxication. nitrogen, creatine kinase, and creatinine levels; arterial blood The initial physiologic effect of cocaine on the cardiovascular gas analysis; urinalysis; and cardiac marker determinations. system is a transient bradycardia as a result of stimulation of the Increased creatine kinase level occurs with rhabdomyolysis. vagal nuclei. Tachycardia typically ensues, predominantly from Cardiac markers are increased in myocardial infarction. Cardiac increased central sympathetic stimulation. Cocaine has a troponin I is preferred to identify acute myocardial13 infarction. cardiostimulatory effect through sensitization to epinephrine A chest radiograph should be obtained in patients with and norepinephrine. -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Verapamil 40Mg, 80Mg, 120Mg and 160Mg Tablets
6 Contents of the pack and other information What Verapamil tablets contain • The active substance is verapamil hydrochloride. Package leaflet: Information for the patient Each tablet contains either 40mg, 80mg, 120mg or 160mg of verapamil hydrochloride. • The other ingredients are croscarmellose Verapamil 40mg, 80mg, 120mg sodium, magnesium stearate, maize starch, and 160mg tablets propylene glycol, sunset yellow aluminium lake (E110), quinoline yellow aluminium lake Read all of this leaflet carefully before you • manage high blood pressure (hypertension), (E104), titanium dioxide (E171), microcrystalline start taking this medicine because it contains used alone or with other drugs for high blood cellulose (E460), hydroxypropylcellulose (E463), pressure. methylhydroxypropylcellulose (E464), purified talc important information for you. • manage and prevent angina. (E553). • Keep this leaflet. You may need to read it again. • treat and prevent certain types of abnormal heartbeats. What Verapamil tablets look like and contents of • If you have any further questions, ask your the pack doctor or pharmacist. 2 What you need to know before you Verapamil 40mg tablets are yellow, circular, biconvex, • This medicine has been prescribed for you film-coated tablets, impressed “C” on one face and only. Do not pass it on to others. It may harm take Verapamil tablets Do not take Verapamil tablets if you: the identifying letters “VR” on the reverse. Tablet them, even if their signs of illness are the • are allergic to verapamil hydrochloride or any of diameter: 6.18-6.82mm. same as yours. the other ingredients of this medicine (listed in Verapamil 80mg tablets are yellow, circular, biconvex • If you get any side effects, talk to your doctor film-coated tablets, impressed “C” on one face and section 6) or pharmacist. -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -

A Unitary Mechanism of Calcium Antagonist Drug Action '(Dihydropyridine/Nifedipine/Verapamil/Neuroleptic/Diltiazem) KENNETH M
Proc. Nati Acad. Sci. USA Vol. 80, pp. 860-864, February 1983 Medical Sciences A unitary mechanism of calcium antagonist drug action '(dihydropyridine/nifedipine/verapamil/neuroleptic/diltiazem) KENNETH M. M. MURPHY, ROBERT J. GOULD, BRIAN L. LARGENT, AND SOLOMON H. SNYDER* Departments of Neuroscience, Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 72S North Wolfe Street, Baltimore, Maryland 21205 Contributed by Solomon H. Snyder, October 21, 1982 ABSTRACT [3H]Nitrendipine binding to drug receptor sites liquid scintillation counting were carried out as described (7). associated with calcium channels is allosterically regulated by a All experiments, performed in triplicate, were replicated at diverse group of calcium channel antagonists. Verapamil, D-600 least three times with similar results. (methoxyverapamit), tiapamil, lidoflazine, flunarizine, cinnari- Guinea pig ileum longitudinal muscles were prepared for zine, and prenylamine all reduce P3H]nitrendipine binding affin- recording as described by Rosenberger et aL (13) and incubated ity. By contrast, diltiazem, a benzothiazepine calcium channel an- in a modified Tyrode's buffer (14) at 370C with continuous aer- tagonist, enhances [3H]nitrendipine binding. All these drugeffects ation with 95% '02/5% CO2. Ileum longitudinal muscles were involve a single site allosterically linked to the [3H]nitrendipine incubated in this buffer for 30 min before Ca2"-dependent con- binding site. Inhibition of t3H]nitrendipine binding by prenyl- were as described Jim et aL amine, lidoflazine, or tiapamil is reversed by D-600and diltiazem, tractions recorded by (15). which alone respectively slightlyreduceorenhance H]mnitrendipine RESULTS binding. Diltiazem reverses the inhibition of [3H]nitrendipine binding by D-600. -

Drugs That Affect the Cardiovascular System
PharmacologyPharmacologyPharmacology DrugsDrugs thatthat AffectAffect thethe CardiovascularCardiovascular SystemSystem TopicsTopicsTopics •• Electrophysiology Electrophysiology •• Vaughn-Williams Vaughn-Williams classificationclassification •• Antihypertensives Antihypertensives •• Hemostatic Hemostatic agentsagents CardiacCardiacCardiac FunctionFunctionFunction •• Dependent Dependent uponupon –– Adequate Adequate amountsamounts ofof ATPATP –– Adequate Adequate amountsamounts ofof CaCa++++ –– Coordinated Coordinated electricalelectrical stimulusstimulus AdequateAdequateAdequate AmountsAmountsAmounts ofofof ATPATPATP •• Needed Needed to:to: –– Maintain Maintain electrochemicalelectrochemical gradientsgradients –– Propagate Propagate actionaction potentialspotentials –– Power Power musclemuscle contractioncontraction AdequateAdequateAdequate AmountsAmountsAmounts ofofof CalciumCalciumCalcium •• Calcium Calcium isis ‘glue’‘glue’ that that linkslinks electricalelectrical andand mechanicalmechanical events.events. CoordinatedCoordinatedCoordinated ElectricalElectricalElectrical StimulationStimulationStimulation •• Heart Heart capablecapable ofof automaticityautomaticity •• Two Two typestypes ofof myocardialmyocardial tissuetissue –– Contractile Contractile –– Conductive Conductive •• Impulses Impulses traveltravel throughthrough ‘action‘action potentialpotential superhighway’.superhighway’. A.P.A.P.A.P. SuperHighwaySuperHighwaySuperHighway •• Sinoatrial Sinoatrial node node •• Atrioventricular Atrioventricular nodenode •• Bundle Bundle ofof -

Tricyclic Antidepressant Overdose
Tricyclic Antidepressant Overdose R. Kevin Smith, DO, and Karen O'Mara, DO Chicago, Illinois Overdose from tricyclic antidepressants (TCAs) is increasing. TCAs are well absorbed orally, highly protein bound, and high ly lipid soluble. Clinical features of poisoning with TCAs occur within 12 hours of ingestion, usually after a dose of 20 mg/kg or more. Clinical symptomatology involves various anticholiner gic, central nervous system, and cardiovascular effects. Car diovascular toxicity accounts for the majority of the fatalities and may include a hyperdynamic response, various arrhyth mias and heart blocks, or severe hypotension. Prolongation of the QRS interval of 10 msec or more implies severe toxicity. Many factors limit the usefulness of drug levels in the over dosed patient. Treatment revolves around good supportive care and general poisoning management. The physician should no longer use physostigmine precipitously. Sodium bicarbon ate is effective in treating many of the cardiovascular compli cations. Other cardiac drugs are used but with varying effi cacy. Patients with significant signs or symptoms of toxicity require monitored hospitalization until clinically free of mani festations for 24 to 48 hours. Overdosage with tricyclic antidepressant drugs sion, coma with respiratory arrest, convulsions, has been reported as early as 1959, one year after complex arrhythmias, and myocardial depression. their introduction to psychiatry. Because of the The purpose of this paper is to review the current complex central and peripheral toxic effects of body of knowledge on tricyclic antidepressants these drugs, the physician who treats the over (TCAs) and their toxicity. Increased understand dosed patient may find himself managing hypoten- ing of the mechanisms of toxicity, pharmacologic interactions, and clinical manifestations allows the outline of a rational mode of therapy for patients who present with TCA overdose. -
Medication-Guide.Pdf
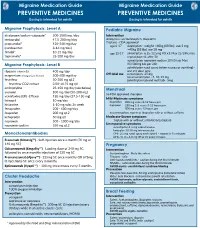
Migraine Medication Guide Migraine Medication Guide PREVENTIVE MEDICINES PREVENTIVE MEDICINES Dosing is intended for adults Dosing is intended for adults Migraine Prophylaxis: Level A Pediatric Migraine divalproex/sodium valproate* 500-1000 mg /day Intervention metoprolol 47.5-200 mg/day Analgesics: acetominophen, ibuprofen propranolol* 120-240 mg/day Triptans - FDA approved age 6-17 rizatriptan - weight <40kg (88 lbs), use 5 mg; (candesartan 8-16 mg/day) >40kg (88 lbs) use 10 mg timolol* 10-15 mg/day age 12-17 almotriptan- 6.25-12.5mg PO x1; Max 25/24h; may topiramate* 25-200 mg/day repeat dose x1 after 2h sumatriptan-naproxen sodium 10/60 tab; Max Migraine Prophylaxis: Level B 85/500mg tab per 24h zolmitriptan nasal spray (when nausea or vomiting) - riboavin (vitamin B2) 400 mg/day one 2.5 dose only Off-label use sumatriptan- 25 mg magnesium trimagnesium dicitrate 500-600 mg/day nasal sumatriptan - 5, 10, 25 mg feverfew 50-300 mg q12 zolmitriptan tab and melt tab - 5mg feverfew CO2 extract 2.08-18.75 mg q8 amitriptyline 25-150 mg/day (see below) Menstrual atenolol 100 mg/day (50-200 mg) no FDA approved therapies venlafaxine (ER) -Effexor 150 mg/day (37.5-150 mg) Mild-Moderate symptoms lisinopril 10 mg/day ibuprofen 800 mg every 8-12 hours prn histamine 1-10 mg subc 2x week naproxen 250 mg 1-2 every 8-12 hours prn fenoprofen 200 - 600 mg/day 500 mg every 12 hours prn acetaminophen, aspirin or ibuprofen with or without caffeine ibuprofen 200 mg q12 ketoprofen 50 mg q8 Moderate-Severe symptoms naproxen 500 - 1000 mg/day triptan with or -

Cocaine and the Heart M Egred, G K Davis
568 Postgrad Med J: first published as 10.1136/pgmj.2004.028571 on 2 September 2005. Downloaded from REVIEW Cocaine and the heart M Egred, G K Davis ............................................................................................................................... Postgrad Med J 2005;81:568–571. doi: 10.1136/pgmj.2004.028571 Cocaine is the second commonest illicit drug used and the N Genitourinary and obstetric: renal and testi- cular infarction, abruptio placentae, sponta- most frequent cause of drug related deaths. Its use is neous abortion, prematurity, and growth associated with both acute and chronic complications that retardation.9 may involve any system, the most common being the N Neurological: seizures, migraine, cerebral 10 cardiovascular system. Cocaine misuse has a major effect infarction, and intracranial haemorrhage. N Musculoskeletal and dermatological: rhabdo- in young adult drug users with resulting loss of productivity myolysis, skin ischaemia, superficial and deep and undue morbidity with cocaine related cardiac and venous thrombosis, and thrombophlebitis.11 cerebrovascular effects. Many cocaine users have little or In this review we will focus on the cardiovas- no idea of the risks associated with its use. Patients, health cular effects of cocaine. care professionals, and the public should be educated about the dangers and the considerable risks of cocaine PHARMACOLOGY OF COCAINE use. This review concentrates on the cardiovascular effects Cocaine (benzoylmethylecgonine, C17,H21,NO4) is an alkaloid extract from the leaf of the of cocaine and their management. Erythroxylon coca plant, which usually grows in ........................................................................... South America. It is available in two forms: N Hydrochloride salt: prepared by dissolving the alkaloid in hydrochloric acid forming a water ocaine is the second commonest illicit drug soluble powder or granule that decomposes used and the most frequent cause of drug when heated. -

Va / Dod Depression Practice Guideline Provider Care Card Antidepressant
VA / DOD DEPRESSION PRACTICE GUIDELINE PROVIDER CARE CARD CARD ANTIDEPRESSANT MEDICATION TABLE 7 Refer to pharmaceutical manufacturer’s literature for full prescribing information SEROTONIN SELECTIVE REUPTAKE INHIBITORS (SSRIs) GENERIC BRAND NAME ADULT STARTING DOSE MAX EXCEPTION SAFETY MARGIN TOLERABILITY EFFICACY SIMPLICITY Citalopram Celexa 20 mg 60 mg Reduce dose Nausea, insomnia, for the elderly & No serious systemic sedation, Fluoxetine Prozac 20 mg 80 mg those with renal toxicity even after headache, fatigue or hepatic substantial overdose. dizziness, sexual AM daily dosing. Paroxetine Paxil 20 mg 50 mg failure Drug interactions may dysfunction Response rate = Can be started at Sertraline Zoloft 50 mg 200 mg include tricyclic anorexia, weight 2 - 4 wks an effective dose antidepressants, loss, sweating, GI immediately. First Line Antidepressant Medication carbamazepine & distress, tremor, Drugs of this class differ substantially in safety, tolerability and simplicity when used in patients warfarin. restlessness, on other medications. Can work in TCA (tricyclic antidepressant) nonresponders. Useful in agitation, anxiety. several anxiety disorders. Taper gradually when discontinuing these medications. SEROTONIN and NOREPHINEPHRINE REUPTAKE INHIBITORS GENERIC BRAND NAME ADULT STARTING DOSE MAX EXCEPTION SAFETY MARGIN TOLERABILITY EFFICACY SIMPLICITY Reduce dose Take with food. Venlafaxine IR Effexor IR 75 mg 375 mg for the elderly & Comparable to BID or TID No serious systemic those with renal SSRIs at low dose. dosing with IR. toxicity. or hepatic Nausea, dry mouth, Response rate = Daily dosing Downtaper slowly to Venlafaxine XR Effexor XR 75 mg 375 mg failure insomnia, anxiety, 2 - 4 wks with XR. prevent clinically somnolence, head (4 - 7 days at Can be started at significant withdrawal ache, dizziness, ~300 mg/day) an effective dose Dual action drug that predominantly acts like a Serotonin Selective Reuptake inhibitor at low syndrome. -

Drug/Substance Trade Name(S)
A B C D E F G H I J K 1 Drug/Substance Trade Name(s) Drug Class Existing Penalty Class Special Notation T1:Doping/Endangerment Level T2: Mismanagement Level Comments Methylenedioxypyrovalerone is a stimulant of the cathinone class which acts as a 3,4-methylenedioxypyprovaleroneMDPV, “bath salts” norepinephrine-dopamine reuptake inhibitor. It was first developed in the 1960s by a team at 1 A Yes A A 2 Boehringer Ingelheim. No 3 Alfentanil Alfenta Narcotic used to control pain and keep patients asleep during surgery. 1 A Yes A No A Aminoxafen, Aminorex is a weight loss stimulant drug. It was withdrawn from the market after it was found Aminorex Aminoxaphen, Apiquel, to cause pulmonary hypertension. 1 A Yes A A 4 McN-742, Menocil No Amphetamine is a potent central nervous system stimulant that is used in the treatment of Amphetamine Speed, Upper 1 A Yes A A 5 attention deficit hyperactivity disorder, narcolepsy, and obesity. No Anileridine is a synthetic analgesic drug and is a member of the piperidine class of analgesic Anileridine Leritine 1 A Yes A A 6 agents developed by Merck & Co. in the 1950s. No Dopamine promoter used to treat loss of muscle movement control caused by Parkinson's Apomorphine Apokyn, Ixense 1 A Yes A A 7 disease. No Recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. It is often claimed that BZP was originally Benzylpiperazine BZP 1 A Yes A A synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals.