Proximal and Distal Convoluted Tubules Histology > Urogenital System > Urogenital System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Control of Fluid Absorption in the Renal Proximal Tubule
Control of fluid absorption in the renal proximal tubule Maurice B. Burg, Jack Orloff J Clin Invest. 1968;47(9):2016-2024. https://doi.org/10.1172/JCI105888. Research Article Glomerulotubular balance was investigated in isolated, perfused rabbit proximal tubules in vitro in order to evaluate some of the mechanisms proposed to account for the proportionate relationship between glomerular filtration rate and fluid absorption generally observed in vivo. The rate of fluid transport from lumen to bath in proximal convoluted tubules in vitro was approximately equal to the estimated normal rate in vivo. The absorption rate in proximal straight tubules however was approximately one-half as great. If the mechanism responsible for maintenance of glomerulotubular balance is intrinsic to the proximal tubule, as has been proposed on the basis of micropuncture studies, the rate of fluid absorption in vitro should be directly related to the perfusion rate and/or tubule volume. In the present studies absorption rate was only minimally affected when perfusion rate was increased or the tubule distended. Thus, glomerulotubular balance is not mediated by changes in velocity of flow of the tubular fluid or tubular diameter and therefore is not an intrinsic property of the proximal tubule. It has also been proposed that glomerulotubular balance results from a humoral feedback mechanism in which angiotensin directly inhibits fluid absorption by the proximal convoluted tubule. In the present experiments, angiotensin was found to have no significant effect on absorption rate. Find the latest version: https://jci.me/105888/pdf Control of Fluid Absorption in the Renal Proximal Tubule MAURICE B. -
![L5 6 -Renal Reabsorbation and Secretation [PDF]](https://docslib.b-cdn.net/cover/2118/l5-6-renal-reabsorbation-and-secretation-pdf-252118.webp)
L5 6 -Renal Reabsorbation and Secretation [PDF]
Define tubular reabsorption, Identify and describe tubular secretion, Describe tubular secretion mechanism involved in transcellular and paracellular with PAH transport and K+ Glucose reabsorption transport. Identify and describe Identify and describe the Study glucose titration curve mechanisms of tubular characteristic of loop of in terms of renal threshold, transport & Henle, distal convoluted tubular transport maximum, Describe tubular reabsorption tubule and collecting ducts splay, excretion and filtration of sodium and water for reabsorption and secretion Identify the tubular site and Identify the site and describe Revise tubule-glomerular describe how Amino Acids, the influence of aldosterone feedback and describe its HCO -, P0 - and Urea are on reabsorption of Na+ in the physiological importance 3 4 reabsorbed late distal tubules. Mind Map As the glomerular filtrate enters the renal tubules, it flows sequentially through the successive parts of the tubule: The proximal tubule → the loop of Henle(1) → the distal tubule(2) → the collecting tubule → finally ,the collecting duct, before it is excreted as urine. A long this course, some substances are selectively reabsorbed from the tubules back into the blood, whereas others are secreted from the blood into the tubular lumen. The urine represent the sum of three basic renal processes: glomerular filtration, tubular reabsorption, and tubular secretion: Urinary excretion = Glomerular Filtration – Tubular reabsorption + Tubular secretion Mechanisms of cellular transport in the nephron are: Active transport Pinocytosis\ Passive Transport Osmosis “Active transport can move a solute exocytosis against an electrochemical gradient and requires energy derived from metabolism” Water is always reabsorbed by a Simple diffusion passive (nonactive) (Additional reading) Primary active (without carrier physical mechanism Secondary active The proximal tubule, reabsorb protein) called osmosis , transport large molecules such as transport Cl, HCO3-, urea , which means water proteins by pinocytosis. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Novel Tubular–Glomerular Interplay in Diabetic Kidney Disease Mediated
Clinical and Experimental Nephrology https://doi.org/10.1007/s10157-019-01719-4 INVITED REVIEW ARTICLE Novel tubular–glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide Oshima Award Address 2017 Kazuhiro Hasegawa1 Received: 6 December 2018 / Accepted: 15 February 2019 © The Author(s) 2019 Abstract Tubules interact with glomeruli, which are composed of podocytes, parietal epithelial cells, mesangial cells, and glomerular endothelial cells. Glomerular–tubular balance and tubuloglomerular feedback are the two components of the tubular–glo- merular interplay, which has been demonstrated to play roles in physiological renal function and in diabetic kidney disease (DKD), in which proteins leaking from glomeruli arrive at tubular regions, leading to further tubular injury caused by the accumulation of proteinuria-inducing reactive oxygens species and various cytokines. In the current review, we present our recent work identifying a novel tubular–glomerular interplay in DKD mediated by sirtuin 1 and nicotinamide mononucleotide. Keywords Sirtuin 1 · Tubuloglomerular feedback · Diabetic kidney disease · Nicotinamide mononucleotide Introduction The longevity gene sirtuin 1 In this review, we summarize our studies revealing the novel We have demonstrated the role of SIRT1 in kidneys, par- roles of sirtuin 1 (SIRT1) and nicotinamide mononucleo- ticularly in DKD. Figure 1 outlines the basic characteristics tide (NMN) in the tubular–glomerular interplay in diabetic of SIRT1, one of the seven isoforms of mammalian sirtuins, kidney disease (DKD). First, we overview the basic func- which are found in specific intracellular compartments. tions of SIRT1 and NMN and changes i1 and NMN during The first sirtuin that was discovered was Sir2 in yeast [1]. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -

Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease
Diabetes Volume 66, April 2017 791 Richard E. Gilbert Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease Diabetes 2017;66:791–800 | DOI: 10.2337/db16-0796 The current view of diabetic kidney disease, based on estimated glomerular filtration rate (eGFR) decline (2). In meticulously acquired ultrastructural morphometry and recognition of these findings, the term diabetic kidney the utility of measuring plasma creatinine and urinary al- disease rather than diabetic nephropathy is now commonly bumin, has been almost entirely focused on the glomer- used. On the background of recent advances in the role of ulus. While clearly of great importance, changes in the the proximal tubule as a prime mover in diabetic kidney PERSPECTIVES IN DIABETES glomerulus are not the major determinant of renal prog- pathology, this review highlights key recent developments. nosis in diabetes and may not be the primary event in the Published mostly in the general scientific and kidney- development of diabetic kidney disease either. Indeed, specific literature, these advances highlight the pivotal advances in biomarker discovery and a greater appreci- role this part of the nephron plays in the initiation, pro- ation of tubulointerstitial histopathology and the role of gression, staging, and therapeutic intervention in diabetic tubular hypoxia in the pathogenesis of chronic kidney kidney disease. From a pathogenetic perspective, as illus- disease have given us pause to reconsider the current trated in Fig. 1 and as elaborated on further in this review, “glomerulocentric” paradigm and focus attention on the proximal tubule that by virtue of the high energy require- tubular hypoxia as a consequence of increased energy de- ments and reliance on aerobic metabolism render it par- mands and reduced perfusion combine with nonhypoxia- ticularly susceptible to the derangements of the diabetic related forces to drive the development of tubular atrophy fi state. -
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -
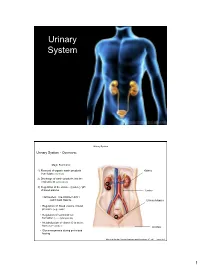
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels