Enolate Anions and Enamines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkylation by Enamines for Synthesis of Some Heterocyclic Compounds ﻴﺩ
------J. Raf. Sci., Vol. 20, No.2, pp 92- 101, 2009 ------ Alkylation by Enamines for Synthesis of some Heterocyclic Compounds Jasim A. Abdullah Department of Chemistry College of Education Mosul University (Received 25/ 9/ 2008 ; Accepted 13 / 4 / 2009) ABSTRACT Compounds of 4-phenyl-3-butene-2-one (1) and 4-(4-chlorophenyl)-3-butene-2-one (2) were prepared by reaction of benzaldehyde or 4-chlorobenzaldehyde with acetone. Also 1,3-diphenyl-2-chloropropene-1-one (3) was synthesized from the reaction of benzaldehyde with 2-chloroacetophenone through Claisen-Shmidt condensation. The substituted ∆1(9)- octalone-2 (4,5) was prepared by reaction of the compounds (1and2) with cyclohexanone through Michael addition followed by aldol condensation. Compounds (4,5) were reacted with alkyl halide, as 2-chloroacetophenone or 1,3-diphenyl-2-chloropropene-1-one (3), via enamines formation which then hydrolyzed to give compounds (6-9). Compounds (6,7) were reacted with hydrazine , urea and thiourea to afford compounds (10-15) . The structures of all synthesized compounds were confirmed by available physical and spectral means . Keywords : Enamines , Heterocyclic compounds. ــــــــــــــــــــــــــــــــــــــــــــــــــ ﺍﻻﻟﻜﻠﺔ ﺒﻭﺍﺴﻁﺔ ﺍﻷﻴﻨﺎﻤﻴﻨﺎﺕ ﻟﺘﺸﻴﻴﺩ ﺒﻌﺽ ﺍﻟﻤﺭﻜﺒﺎﺕ ﺍﻟﺤﻠﻘﻴﺔ ﻏﻴﺭ ﺍﻟﻤﺘﺠﺎﻨﺴﺔ ﺍﻟﻤﻠﺨﺹ ﺘﻡ ﺘﺤﻀﻴﺭ ﺍﻟﻤﺭﻜﺒﺎﺕ 4-ﻓﻨﻴل-3-ﺒﻴﻭﺘﻴﻥ-2-ﺍﻭﻥ (1) ﻭ4-(4-ﻜﻠﻭﺭﻭﻓﻨﻴل)-3-ﺒﻴﻭﺘﻴﻥ-2-ﺍﻭﻥ (2) ﻤﻥ ﺘﻔﺎﻋل ﺍﻟﺒﻨﺯﺍﻟﺩﻴﻬﻴﺩ ﺃﻭ 4-ﻜﻠﻭﺭﻭﺍﻟﺒﻨﺯﺍﻟﺩﻴﻬﻴﺩ ﻤﻊ ﺍﻻﺴﻴﺘﻭﻥ . ﺤﻀﺭ ﺍﻟﻤﺭﻜﺏ 3,1-ﺜﻨﺎﺌﻲ ﻓﻨﻴل -2-ﻜﻠـﻭﺭﻭﺒﺭﻭﺒﻴﻥ -1-ﺍﻭﻥ (3) ﻤـﻥ ﺘﻔﺎﻋـل ﺍﻟﺒﻨﺯﺍﻟﺩﻴﻬﻴـﺩ ﻤـﻊ -2 9 1 ﻜﻠﻭﺭﻭﺍﺴﻴﺘﻭﻓﻴﻨﻭﻥ ﺒﻭﺴﺎﻁﺔ ﺘﻜﺎﺜﻑ (ﻜﻠﻴﺯﻥ-ﺸﻤﺩﺕ).ﺤﻀﺭﺕ ﻤﻌﻭﻀﺎﺕ ∆ ( ) –ﺍﻭﻜﺘﺎﻟﻭﻥ-2 ﻤﻥ ﺨﻼل ﺘﻔﺎﻋل ﺍﻟﻤﺭﻜﺒﻴﻥ (2,1) ﻤﻊ ﺍﻟﻬﻜﺴﺎﻨﻭﻥ ﺍﻟﺤﻠﻘﻲ ﺒﻭﺴﺎﻁﺔ ﺍﻀﺎﻓﺔ ﻤﺎﻴﻜل ﺍﻟﺘﻲ ﻴﺘﺒﻌﻬﺎ ﺘﻜﺎﺜﻑ ﺍﻻﻟﺩﻭل . -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

S41467-018-07534-X.Pdf
ARTICLE DOI: 10.1038/s41467-018-07534-x OPEN Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO Xiaoming Jie1, Yaping Shang1, Zhe-Ning Chen 1, Xiaofeng Zhang1, Wei Zhuang1 & Weiping Su1 Enamine and imine represent two of the most common reaction intermediates in syntheses, and the imine intermediates containing α-hydrogen often exhibit the similar reactivity to 1234567890():,; enamines due to their rapid tautomerization to enamine tautomers. Herein, we report that the minor structural difference between the enamine and the enamine tautomer derived from imine tautomerization results in the different chemo- and regioselectivity in the reaction of cyclohexanones, amines and TEMPO: the reaction of primary amines furnishes the formal oxygen 1,2-migration product, α-amino-enones, while the reaction of secondary amines under similar conditions generates exclusively arylamines via consecutive dehydrogenation on the cyclohexyl rings. The 18O-labeling experiment for α-amino-enone formation revealed that TEMPO served as oxygen transfer reagent. Experimental and computational studies of reaction mechanisms revealed that the difference in chemo- and regioselectivity could be ascribed to the flexible imine-enamine tautomerization of the imine intermediate con- taining an α-hydrogen. 1 State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou 350002, China. -
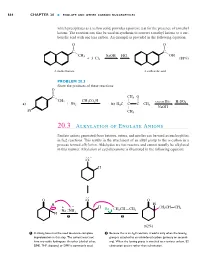
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3. -

Enamine-Based Organocatalysis with Proline And
Acc. Chem. Res. 2004, 37, 580-591 carbon bond in a stereospecific fashion. These same Enamine-Based Organocatalysis features are typically absent in traditional synthetic asym- with Proline and Diamines: The metric aldol methodologies.2,3 Our ideal synthetic catalyst would be one that could Development of Direct Catalytic generate enamines from any number of aldehydes or ketones and then direct bond-forming reactions with a Asymmetric Aldol, Mannich, wide variety of electrophiles beyond the carbonyl elec- Michael, and Diels-Alder trophiles of the aldol reaction. Further, since these cata- lysts also generate imines as part of their reaction mech- Reactions anism, electrophilic catalysis might facilitate diverse WOLFGANG NOTZ, FUJIE TANAKA, AND reactions with nucleophiles. Such an antibody might be termed an ªopen-active siteº catalytic antibody. Indeed, CARLOS F. BARBAS, III* The Skaggs Institute for Chemical Biology and the we were able to demonstrate that our aldolase antibodies Departments of Chemistry and Molecular Biology, The could catalyze not only a wide range of intra- and Scripps Research Institute, 10550 North Torrey Pines Road, intermolecular aldol reactions but also decarboxylation La Jolla, California 92037 reactions via imine catalysis and certain Michael reactions Received February 2, 2004 as well.2 Significantly for what would become our studies in organocatalysis, in our search for ªopen-active siteº ABSTRACT antibody catalysis, we studied the potential of aldolase Enamines and imines have long been recognized as key intermedi- antibodies to catalyze the addition of ketones to nitrosty- ates in enzyme catalysis, particularly within a class of enzymes renes in Michael reactions, the addition of ketones to organic chemists would very much like to emulate, the aldolases. -
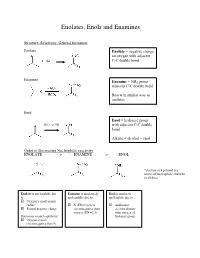
Enolates, Enols and Enamines
Enolates, Enols and Enamines Structure, Reactivity, General formation Enolate Enolate = negative charge on oxygen with adjacent C-C double bond Enamine Enamine = NR2 group adjacent C-C double bond Reacts in similar way as enolates Enol Enol = hydroxyl group - H3O+ or OH with adjacent C-C double bond Alkene + alcohol = enol Order of Decreasing Nucleophilic reactivity ENOLATE > ENAMINE > ENOL *electron-rich pi bond is a source of nucleophilic character in all three Enolate is nucleophilic due Enamine is moderately Enol is moderate to… nucleophilic due to… nucleophile due to… Oxygen’s small atomic radius N (EN=3) is less Additional Formal negative charge electronegative than electron density oxygen (EN =3.5) from oxygen of Detractors to nucleophilicity: hydroxyl group Oxygen is more electronegative than N When looking at which side is favored (products or reactants): Equilibrium favors side with WEAKER acid/base pair Weakest acid and weakest base should be on SAME side When comparing pKa values, juxtapose just the acids or just the bases Side with larger pka value (weaker acid) favored in equilibrium Remember that differences in pKa values reflect a difference quantity of 10x Example: compound H-A (pKa=3) + base compound A (pKa=10) + H-base 7 equilibrium lies to the RIGHT by factor of 10 Some Useful pKa values (from more acidic basic) H2SO4 (pka = -9) H2O (pka = 15.7) + H3O (pka = -1.8) CH3COCH3 (pka = 19) B-diketone (pka = 9) CH3COOCH3 (pKa = 25) HCN (pka = 9.1) LDA-H (pka = 36) CH3OH (pka = 15.5) How do we know which proton to deprotonate? Deprotonate most acidic proton usually leads to the most stable enolate 1. -

Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) 2) Nucleophilic acyl substitution (carboxylic acid derivatives) 3) The alpha-substitution Reactions occur at the position next to the carbonyl group–the alpha position–and result in the substitution of an α hydrogen atom by some other electrophilic group, E+: O O E+ α H E + H+ 4) The carbonyl condensation Reactions take place when two carbonyl compounds react with each other in such a way that the α carbon of one partner becomes bonded to the carbonyl carbon of the second partner. O H O OH 2 H The key feature of the reactions (3, 4) is that they take place through the formation of either enol or enolate ion intermediates. H O O C C C C 11.1 Keto-Enol Tautomerism The interconversion between the keto and enol forms of carbonyl compounds is a special kind of isomerism called tautomerism. In Greek, tauto means “the same,” and meros means “part.” The interconversion of tautomers involves the movement of atoms (H), so they are different from resonance forms. H O Rapid O α H Keto tautomer Enol tautomer 1 Most carbonyl compounds exist almost entirely in the keto form at equilibrium. OOHO OH 99.9999% 0.0001% 99.999999% 0.000001% Mechanism of keto-enol tautomerism Acid catalysis H A H H H O O O A O H H H + HA Keto tautomer Enol tautomer Base catalysis HOH H OOOH OO H + OH Keto tautomer Enol tautomer 11.2 Reactivity of Enols: The Mechanism of Alpha-Substitution Reactions Since their double bonds are electron-rich, enols behave as nucleophiles and react with electrophiles in the same way as alkenes do. -

Enolate Chemistry
Prof. Dr. Burkhard König, Institut für Organische Chemie, Uni Regensburg 1 Enolate Chemistry 1. Some Basics In most cases the equilibrium lies almost completely on the side of the ketone. The ketone tautomer is electrophilic and reacts with nucleophiles: The enol tautomer is nucleophilic and reacts with electrophiles. There are two possible products - enols are ambident nucleophiles: The nucleophilic enol tautomer (and especially the enolate variant) is one of the most important reactive species for C-C bond formation. Treat a ketone with an appropriate base and can get deprotonation at the α-position to form an enolate: Enolates are synthetically much more useful than enols (although they react analogously). Imine anions and eneamines are synthetic equivalents of enolate anions. Li N N H H Imine anion eneamine By knowing the pKa values of the relevant acidic protons it is possible to predict suitable bases for forming the corresponding enolates. Prof. Dr. Burkhard König, Institut für Organische Chemie, Uni Regensburg 2 Some Important pKa Values - - - O O HC N HC3 NO2 H2CN+ 2 + O O pKa ~ 10 - Enolates are nucleophiles and ketones are electrophiles - therefore there is always the potential problem for self condensation. If this is desirable we need to use a base which does not completely deprotonate the carbonyl compound i.e. set up an equilibrium. This is best achieved when the pKa of the carbonyl group and conjugate acid (of the base) are similar: Prof. Dr. Burkhard König, Institut für Organische Chemie, Uni Regensburg 3 pKa (tBuOH) ~ 18. A stronger base. pKa difference of 2. Ratio of ketone to enolate will be of the order 100:1 i.e. -

The Α-Carbon Atom and Its Pka the Inductive Effect of the Carbonyl
Chapter 18: Enols and Enolates O E+ O H Enolate O E carbonyl H O E+ Enol 18.1: The α-Carbon Atom and its pKa O "' " #' !' ! # 126 The inductive effect of the carbonyl causes the α-protons to be more acidic. The negative charge of the enolate ion (the conjugate base of the aldehyde or ketone) is stabilized by resonance delocalization. The pKa of the α-protons of aldehydes and ketones is in the range of 16-20 (Table 18.1, p. 754) resonance effect ! - inductive O O !+ O + B effect C H C C + H-B !+ C C C carbonyl Enolate anion H C C C C C C O O O O H3C CH3 C H3CH2COH H3C CH3 ethane acetone ethanol 127 pKa= 50-60 pKa= 19 pKa= 16 65 O O O O O C C Cl C C C H3C CH3 H3C C H3C C H3C C CH3 H H H H H H pKa 19 14 16 9 O O + H O C + H3O C 2 H C CH H3C CH3 3 2 acid base conjugate conjugate base acid pKa 19 -1.7 (weaker acid) (weaker base) (stronger base) (stronger acid) O O + HO C + H O C H C CH 2 H3C CH3 3 2 acid base conjugate conjugate base acid pKa 19 15.7 (weaker acid) (weaker base) (stronger base) (stronger acid) 128 O O O O C C + H2O C C + HO H C C CH H C C CH 3 3 3 3 H H H acid base conjugate conjugate base acid pKa 9 15.7 (stronger acid) (stronger base) (weaker base) (weaker acid) 18.2: The Aldol Condensation- An enolate of one carbonyl (nucleophile) reacts with the carbonyl carbon (electrophile) of a second carbonyl compound resulting in the formation of a new C-C bond O OH O 2 H C C H + HO + HO 3 H3C CH CH2 C H acetaldehyde 3-hydroxybutanal (!-hydroxy aldehyde) 129 66 Mechanism of the base-catalyzed aldol condensation (Fig. -

Chem 353: Aldol Condensation
ALDOL.1 ORGANIC SYNTHESIS: ALDOL CONDENSATION REACTION TECHNIQUES REQUIRED: Filtration (Vacuum), Recrystallisation, Melting Point Determination, Yield calculation OTHER DOCUMENTS: Experimental Procedure, Report template (pdf), Report template (doc), Spectra INTRODUCTION In an "aldol addition" reaction, an enol or more commonly an enolate of an aldehyde or ketone reacts with a second aldehyde or ketone forming a new carbon-carbon bond. This makes the aldol reaction an important reaction for organic synthesis. Originally, the aldol reaction used ethanal (see below) and therefore the product contained both an aldehyde and an alcohol functional group; thus it became known as the aldol reaction. Recall that aldehydes and ketones typically undergo nucleophilic addition reactions. The aldol reaction demonstrates how carbonyl compounds can react as electrophiles or nucleophiles depending on the reaction conditions and what other species are present. Alcohols are similar in that respect. Can you think of examples to illustrate that ? The aldol reaction requires an aldehyde or ketone that contains at least one -hydrogen (the -hydrogen is on the carbon adjacent to the C=O group) since the -hydrogen is required in order to form the enol or enolate. In the base-catalysed aldol reaction, the relatively acidic hydrogen on the -carbon (typical pKa 16-20) is deprotonated by a base to form the enolate. The enolate reacts as a carbon nucleophile that can then react with the electrophilic carbonyl carbon of another aldehyde or ketone molecule. Depending on the strength of the base used, the extent of deprotonation can be controlled. If a strong base is used (such as lithium diisopropylamide, LDA) then deprotonation is quantitative (100%). -

Paper : HCHE4CC09LTH
Paper : HCHE4CC09LTH Module-1: Exploitation of acidity of α-H of C=O and organometallics Mannich reaction The condensation of a CH-activated compound (usually an aldehyde or ketone) with a primary or secondary amine (or ammonia) and a nonenolizable aldehyde (or ketone) to afford aminoalkylated derivatives is known as the Mannich reaction. More generally it is The t hree-component aminomethylation from amine, aldehyde and a compound with an acidic methylene moiety. Mechanism The mechanism of the Mannich reaction has been extensively investigated. The reaction can proceed under both acidic and basic conditions, but acidic conditions are more common. Under acidic conditions the first step is the reaction of the amine component with the protonated non-enolizable carbonyl compound to give a hemiaminal, which after proton transfer loses a molecule of water to give the electrophilic iminium ion.50 This iminium ion then reacts with the enolized carbonyl compound (nucleophile) at its α-carbon in an aldol-type reaction to give rise to the Mannich base. Mannich reaction Examples Asymmetric Mannich Reaction Perkin Reaction The condensation of aromatic aldehydes with the anhydrides of aliphatic carboxylic acids in the presence of a weak base to afford α,β-unsaturated carboxylic acids is known as the Perkin reaction or Perkin condensation . The general features of the transformation are: 1) The aldehyde component is most often aromatic, but aliphatic aldehydes with no α-hydrogens as well as certain α,β-unsaturated aldehydes can also be used. 2) the reaction is more facile and gives higher yield of the product when the aromatic aldehyde has one or more electron-withdrawing substituents.