Chapter 20 Enolates / Other Carbanions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Direct Visualization of a Mechanochemically Induced Molecular Rearrangement Karen J
Angewandte Communications Chemie How to cite: Angew. Chem. Int. Ed. 2020, 59, 13458–13462 Mechanochemical Synthesis International Edition: doi.org/10.1002/anie.201914921 German Edition: doi.org/10.1002/ange.201914921 Direct Visualization of a Mechanochemically Induced Molecular Rearrangement Karen J. Ardila-Fierro, Stipe Lukin, Martin Etter, Krunoslav Uzˇarevic´, Ivan Halasz, Carsten Bolm, and JosØ G. Hernµndez* Abstract: Recent progress in the field of mechanochemistry (thio)acylations[5]). In this context, we became curious as to has expanded the discovery of mechanically induced chemical whether a combination of in situ real-time monitoring by transformations to several areas of science. However, a general synchrotron powder X-ray diffraction (PXRD), Raman fundamental understanding of how mechanochemical reac- spectroscopy, and temperature sensing could be applied for tions by ball milling occur has remained unreached. For this, the investigation of a fundamentally different type of organic we have now implemented in situ monitoring of a mechano- reaction; namely, a molecular rearrangement. As the first chemically induced molecular rearrangement by synchrotron example, we considered the emblematic benzil–benzilic acid X-ray powder diffraction, Raman spectroscopy, and real-time rearrangement in the presence of hydroxide ions (Sche- temperature sensing. The results of this study demonstrate that me 1a). molecular rearrangements can be accomplished in the solid state by ball milling and how in situ monitoring techniques enable the visualization of changes occurring at the exact instant of a molecular migration. The mechanochemical benzil–benzilic acid rearrangement is the focal point of the study. In mechanochemical reactions by ball milling, collisions of accelerated balls inside a milling container maintain a con- stant mixing of the reactants while simultaneously trans- ducing the energy required to induce specific physicochemical changes into the milled system. -
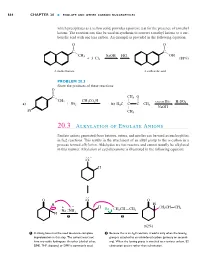
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3. -

A Facile Solvent-Free Cannizzaro Reaction. an Instructional Model for Introductory Organic Chemistry Laboratory
In the Laboratory edited by Green Chemistry Mary M. Kirchhoff ACS Education Division Washington, DC 20036 A Facile Solvent-Free Cannizzaro Reaction An Instructional Model for Introductory Organic Chemistry Laboratory Sonthi Phonchaiya and Bhinyo Panijpan Institute for Innovation and Development of Learning Process, Mahidol University, Bangkok 10400, Thailand Shuleewan Rajviroongit* Department of Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand; *[email protected] Tony Wright School of Education, University of Queensland, 4072, QLD, Australia Joanne T. Blanchfield School of Molecular and Microbial Sciences, University of Queensland, 4072, QLD, Australia To make students in chemistry laboratory more apprecia- 2-chlorobenzyl alcohol by filtration: the solid 2-chlorobenzyl tive of the environmental aspects of chemistry, it is important alcohol retained by the filter was washed twice with water. After that the laboratory exercise address possible environmental im- acidification of potassium 2-chlorobenzoate and filtration, the pacts, for example, energy used, chemical wastes produced, and precipitated 2-chlorobenzoic acid was also rinsed with water. In the use of organic solvents. A reaction that involves only a small both cases small amounts of products were sacrificed, leading to amount(s) of reactants in an aqueous solvent with no organic slightly reduced yields. The reaction is shown in Scheme I. solvent used in the product purification step would result in a Students achieved yields of 35–85% for each pure product. greener chemistry laboratory. A pedagogical method such as They characterized their partially dried products by comparing guided inquiry will make the laboratory more learner-centered the Rf values on TLC with the authentic samples. -

Practical Organic Pharmaceutical Chemistry 4Th Stage (2Nd Course)
Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ Practical Organic Pharmaceutical Chemistry 4th Stage (2nd course) Lab. 1,2 Prepared by: Assist.Lecturer Ali Amjed 2017-2018 1 Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ Cannizzaro Reaction Synthesis of Benzoic Acid and Benzyl Alcohol Both Alcohols and Organic Acids are Well Known for their Biological Actions which include: Antibacterial, Preservatives for Food and Pharmaceutics, Local Antiseptic, Local Anesthetic and Antipruritic. Benzyl Alcohol: Benzyl Alcohol can be Prepared by Hydrolysis of Benzyl Chloride with Sodium Hydroxide as shown below: Benzyl chloride Benzyl alcohol Properties of Benzyl Alcohol: 1) Colorless Liquid. 2) Moderate Solubility in Water. 3) Completely Miscible with Organic Solvents like Alcohol and Diethyl ether. 4) Boiling Point: 204-205 °C. 5) Have Low Vapor Pressure and Low Toxicity. 2 Al-ISRAA University College Pharmacy Department ------------------------------------------------------------------------------------------------------------------------ In Pharmaceutical , Benzyl Alcohol used at Low Concentration as: 1) Antiseptic (Bacterio Static). 2) Local Anesthetic. 3) Preservative for Food Industry. 4) In 10% Ointments is used as Antipruritic. In High Concentration Could Cause Hypotension and Respiratory Failure. Benzoic -

Paper II, Organic Chemistry, Unit IV the Cannizzaro Reaction
B.Sc. Part-1(Hons.), Paper II, Organic Chemistry, unit IV (Study material prepared by Dr. Ruchi Jain, S.B.S.S.College, Begusarai) The Cannizzaro reaction The Cannizzaro reaction is also known as Cannizzaro disproportionation and it is a redox reaction between aromatic aldehydes, formaldehyde or other aliphatic aldehydes without α-hydrogen. Base is used to afford the corresponding alcohols and carboxylic acids.(pathway A) Final deprotonation of the carboxylic acid drives the reaction forward. Pathway B: As you know, aldehydes are generally at least partly hydrated in water. Hydration is catalysed by base, and we can represent the hydration step in base like this. The hydration product is an anion but, if the base is sufficiently strong (or concentrated) and as long as the aldehyde cannot be enolized, at least some will be present as a dianion. The dianion is very unstable, and one way in which it can become much more stable is by behaving like a tetrahedral intermediate. Which is the best leaving group? Out of a choice of O2–, R–, and H–, it’s H– that (if reluctantly) has to go. Hydride is, of course, too unstable to be released into solution but, if there is a suitable electrophile at hand (another molecule of aldehyde, for example), it is transferred to the electrophilic centre in a mechanism that bears some resemblance to a borohydride reduction. The dianion becomes a much more stable carboxylate monoanion, and a second molecule of aldehyde has been reduced to an alcohol. This is the Cannizzaro reaction: in this case it takes the form of a disproportionation of two molecules of aldehyde to one of carboxylate and one of alcohol. -

Aldol Condensation
SEM-III, CORE COURSE-7 ORGANIC CHEMISTRY-3 TOPIC: CARBONYL AND RELATED COMPOUNDS SUB-TOPIC: CONDENSATIONS (PPT-7) Dr. Kalyan Kumar Mandal Associate Professor St. Paul’s C. M. College Kolkata CONDENSATION • ALDOL CONDENSATION • CLAISEN-SCHMIDT REACTION • TOLLENS’ CONDENSATION ALDOL CONDENSATION • Carbonyl compounds, aldehydes and ketones, containing an α-hydrogen atom, in presence of dilute alkali, like NaOH, KOH, Ba(OH)2, etc., or dilute acids, like HCl, H2SO4 undergo a type of reaction in which two molecules of the aldehydes or ketones condense to form an unstable syrupy liquid, aldol. • This reaction is a dimerisation of an aldehyde (or ketone) to a β-hydroxyaldehyde (or β-hydroxyketone) by alpha C-H addition of one reactant molecule to the carbonyl group of the second reactant molecule. FACTS • The product is an aldehyde with a hydroxy (ol) group whose trivial name is aldol. The name aldol is given to the whole class of reactions between enolates (or enols) and carbonyl compounds even if the product is not a hydroxyaldehyde. • The Aldol Condensation leads to the formation of carbon-carbon sigma bond between two carbonyl compounds (same or different), one acting as a nucleophilic species and another as an electrophile. • The rate expression for the aldol reaction at low concentrations of - hydroxide ion is, rate = k [CH3CHO][HO ], showing that the formation of the enolate ion is rate-determining. • The base catalysed aldol condensation of acetaldehyde takes place in two steps, the first step being the formation of the carbanion (I), and the second, the combination of this anion with a second molecule of acetaldehyde to form the anion (II) of the aldol. -

Transfer Hydrogenations and Kinetic Resolutions Van Dirk Klomp
transferhydrogenations and kineticresolutions DirkKlomp transferhydrogenationsandkineticresolutions DirkKlomp Uitnodiging voorhetbijwonenvande openbareverdedigingvan hetproefschriften destellingenop maandag 13maart2006 13.00uur endedaaraan voorafgaandetoelichting voorniet-chemiciom 12.30uur indeSenaatszaalvande TechnischeUniversiteitDelft Mekelweg5teDelft Naafloopvande plechtigheidbentuookvan hartewelkomopdereceptie inhetzelfdegebouw. DirkKlomp Esdoornlaan32 1521EBWormerveer 075-6223403 [email protected] Stellingen behorende bij het proefschrift transfer hydrogenations and kinetic resolutions van Dirk Klomp 1- De door Kao et al. gebruikte structuurbepalende verbinding fructose voor de synthese van de mesoporeuze silica SBA-1 bepaalt niet de structuur van het materiaal. H.-M. Kao, C.-C. Ting, A. S. T. Chiang, C.-C. Teng, C.-H. Chen, Chem. Commun. 2005 ,1058-1060. 2- Het door Kim et al. gesuggereerde mechanisme voor de deactivering van het enzym α-CT in de hydrolyse van β-lactonen, waarbij het lacton opent op de 4-positie, is zeer onwaarschijnlijk. D. H. Kim, J. Park, S. J. Chung, J. D. Park, N.-K. Park, J. H. Han, Bioorg. Med. Chem. 2002 , 10 , 2553-2560. 3- Psychologisch gezien betekent de omschakeling door wetenschappelijke tijdschriften van papieren versies naar elektronische, een verlies aan kennis voor de wetenschapper. 4- Omdat de aarde langzamer gaat draaien is soms de toevoeging van schrikkelsecondes noodzakelijk. Wetenschappers die deze secondes pas willen toevoegen op het moment dat ze zijn opgespaard tot een schrikkeluur, omdat de toevoegingen storingen kunnen veroorzaken in elektronische apparatuur, hebben de menselijke factor uit het oog verloren. 5- In de weekeindes waarin een nieuw boek over Harry Potter uitkomt, is het aantal kinderen dat op de eerstehulpafdeling van het ziekenhuis belandt bijna half zo groot als in andere weekeindes. Gwilym et al. hebben de afname over de lange termijn waarschijnlijk onderschat. -
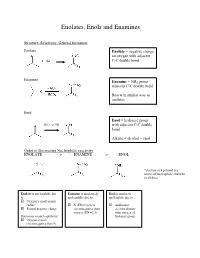
Enolates, Enols and Enamines
Enolates, Enols and Enamines Structure, Reactivity, General formation Enolate Enolate = negative charge on oxygen with adjacent C-C double bond Enamine Enamine = NR2 group adjacent C-C double bond Reacts in similar way as enolates Enol Enol = hydroxyl group - H3O+ or OH with adjacent C-C double bond Alkene + alcohol = enol Order of Decreasing Nucleophilic reactivity ENOLATE > ENAMINE > ENOL *electron-rich pi bond is a source of nucleophilic character in all three Enolate is nucleophilic due Enamine is moderately Enol is moderate to… nucleophilic due to… nucleophile due to… Oxygen’s small atomic radius N (EN=3) is less Additional Formal negative charge electronegative than electron density oxygen (EN =3.5) from oxygen of Detractors to nucleophilicity: hydroxyl group Oxygen is more electronegative than N When looking at which side is favored (products or reactants): Equilibrium favors side with WEAKER acid/base pair Weakest acid and weakest base should be on SAME side When comparing pKa values, juxtapose just the acids or just the bases Side with larger pka value (weaker acid) favored in equilibrium Remember that differences in pKa values reflect a difference quantity of 10x Example: compound H-A (pKa=3) + base compound A (pKa=10) + H-base 7 equilibrium lies to the RIGHT by factor of 10 Some Useful pKa values (from more acidic basic) H2SO4 (pka = -9) H2O (pka = 15.7) + H3O (pka = -1.8) CH3COCH3 (pka = 19) B-diketone (pka = 9) CH3COOCH3 (pKa = 25) HCN (pka = 9.1) LDA-H (pka = 36) CH3OH (pka = 15.5) How do we know which proton to deprotonate? Deprotonate most acidic proton usually leads to the most stable enolate 1. -

Organic Chemistry Reactions
Organic Chemistry Reactions 1 Birch Reduction Birch Reduction The Birch Reduction offers access to substituted 1,4-cyclohexadienes. Mechanism of Birch Reduction I) The question of why the 1,3-diene is not formed, even though it would be more stable through conjugation, can be rationalized with a simple mnemonic. When viewed in valence bond terms, electron-electron repulsions in the radical anion will preferentially have the nonbonding electrons separated as much as possible, in a 1,4-relationship This question can also be answered by considering the mesomeric structures of the dienyl carbanion: II) The numbers, which stand for the number of bonds, can be averaged and compared with the 1,3- and the 1,4-diene. The structure on the left is the average of all mesomers depicted above followed by 1,3 and 1,4-diene: III) The difference between the dienyl carbanion and 1,3-diene in absolute numbers is 2, and between the dienyl carbanion and 1,4-diene is 4/3. The comparison with the least change in electron distribution will be preferred. Reactions of arenes with +I- and +M-substituents lead to the products with the most highly substituted double bonds: IV) The effect of electron-withdrawing substituents on the Birch Reduction varies. For , the reaction of benzoic acid leads to 2,5- cyclohexadienecarboxylic acid, which can be rationalized on the basis of the carboxylic acid stabilizing an adjacent anion: V) Alkene double bonds are only reduced if they are conjugated with the arene, and occasionally isolated terminal alkenes will be reduced. 2 Aldol Condensation Aldol Condensation In some cases, the adducts obtained from the Aldol Addition can easily be converted (in situ) to ,-unsaturated carbonyl compounds, either thermally or under acidic or basic catalysis. -

Aldol Reaction Clayden.Pdf
Reactions of enolates with aldehydes and ketones: the aldol reaction 27 Connections Building on: Arriving at: Looking forward to: • Carbonyl compounds reacting with • Reactions with carbonyl compounds • Enolates taking part in a substitution cyanide, borohydride, and bisulfite as both nucleophile and electrophile at C=O ch28 nucleophiles ch6 • How to make hydroxy-carbonyl • Enolates undergoing conjugate • Carbonyl compounds reacting with compounds or enones by the aldol addition ch29 organometallic nucleophiles ch9 reaction • Synthesis of aromatic heterocycles • Carbonyl compounds taking part in • How to be sure that you get the ch44 nucleophilic substitution reactions product you want from an aldol • Asymmetric synthesis ch45 ch12 & ch14 reaction • Biological organic chemistry • How enols and enolates react with • The different methods available for ch49–ch51 heteroatomic electrophiles such as doing aldol reactions with enolates of + Br2 and NO ch21 aldehydes, ketones, and esters • How enolates and their equivalents • How to use formaldehyde as an react with alkylating agents ch26 electrophile • How to predict the outcome of intramolecular aldol reactions Introduction: the aldol reaction The simplest enolizable aldehyde is acetaldehyde (ethanal, CH3CHO). What happens if we add a small amount of base, say NaOH, to this aldehyde? Some of it will form the enolate ion. O O O NaOH HO H H H H acetaldehyde enolate ion Only a small amount of the nucleophilic enolate ion is formed: hydroxide is not basic enough to enolize an aldehyde completely. Each molecule of enolate is surrounded by molecules of the alde- hyde that are not enolized and so still have the electrophilic carbonyl group intact. Each enolate ion will attack one of these aldehydes to form an alkoxide ion, which will be protonated by the water molecule formed in the first step.