The Α-Carbon Atom and Its Pka the Inductive Effect of the Carbonyl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ochem ACS Review 18 Enols and Enolates
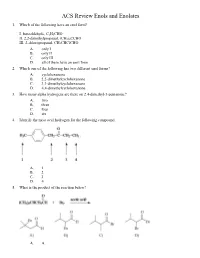
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Lecture 6 the Crossed Aldol Reaction and Its Many Variants
Lecture 6 The Crossed Aldol Reaction and its Many Variants Objectives: By the end of this lecture you will be able to: 1. make an appropriate choice of base to completely enolise carbonyl compounds; 2. use enolates in a crossed aldol reaction; 3. recognise the aldol functional group motif, and its variants, in complex molecules; 4. use the aldol disconnection to simplify a retrosynthetic analysis; 5. use the Reformatski and Mukaiyama aldol reactions in synthesis; 6. draw arrow-pushing mechanisms for all the aldol reactions discussed in this lecture. Introduction We have seen how choosing a base (B-) whose conjugate acid (B−H) is a much poorer acid − i.e. has a much higher pKa − than the proton we wish to abstract, ensures effectively complete deprotonation of the α-C−H of a carbonyl compound to provide the corresponding enolate. By completely enolising the carbonyl group, we can suppress self-condensation aldol processes, and instead use a different electrophile such as an aldehyde to form a β-hydroxy carbonyl compound. This reaction sequence is identical in basic mechanism to the intramolecular aldol processes that we discussed in previous lectures. It is now described as a crossed aldol condensation because the electrophile is a different carbonyl compound to the one that was used to form the nucleophilic enolate. This reaction, and its many variants, provides one of the most important methods for preparing C−C bonds. Pattern Recognition The aldol reaction and its many variants are very useful reactions in synthesis. You need to be able to identify the patterns or functional group motifs where this type of bond-forming process can be used. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -
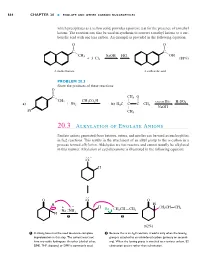
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3. -

MINDO-Forces Study on the Substituent Effect in the Keto-Enol Tautomerism of Acetyl Derivatives
MINDO-Forces Study on the Substituent Effect in the Keto-Enol Tautomerism of Acetyl Derivatives Wasim F. Al-Halasah, Ali Mahasnah, and Salim M. Khalil Chemistry Department, College of Science, University of Mutah, Karak, Jordan Reprint requests to Prof. S.M.K.; E-mail: [email protected] Z. Naturforsch. 59a, 299 – 308 (2004); received February 11, 2004 MINDO-Forces calculations with complete geometry optimization have been performed on ac- etaldehyde, vinyl alcohol and acetyl derivatives CH3COX(X=H, F, OH, CN, NH2,NO2,CH3, CF3,OCH3). It was found that acetaldehyde is more stable than vinyl alcohol by 10.451 kcal/mol. Thermodynamically, keto tautomers are more stable than their enol counterparts. This agrees with theoretical calculations. The electron releasing substituents tend to stabilize keto tautomers, while the electron withdrawing substituents tend to destabilize the keto tautomers, relative to the parent. Geometrical parameters, heats of formation, electron densities, Gibbs free energies and orbital ener- gies (HOMO-LUMO) are reported. Key words: Acetaldehyde; Vinyl Alcohol; Keto-Enol Tautomerism; Acetyl Derivative. 1. Introduction 311++G∗∗//MP2(full)/6-31G∗ levels of theory, ac- etaldehyde 1 is found to lie 11.2 and 13.35 kcal/mol Tautomerism refers to the equilibrium between two below vinyl alcohol 2 on the potential energy sur- different structures of the same compound. It is a pro- face, respectively. Recent B3LYP and G2MP2 calcu- totropic rearrangement in which a hydrogen at the α- lations [14] show that acetaldehyde 1 lies 10.4 and position to a carbon-heteroatom double bond migrates 11.1 kcal/mol below vinyl alcohol 2, respectively. -

Determination of Solvent Effects on Ketoðenol Equilibria of 1,3-Dicarbonyl Compounds Using NMR: Revisiting a Classic Physical C
In the Laboratory Determination of Solvent Effects on Keto–Enol Equilibria W of 1,3-Dicarbonyl Compounds Using NMR Revisiting a Classic Physical Chemistry Experiment Gilbert Cook* and Paul M. Feltman Department of Chemistry, Valparaiso University, Valparaiso, IN 46383; *[email protected] “The influence of solvents on chemical equilibria was discovered in 1896, simultaneously with the discovery of keto–enol tautomerism in 1,3-dicarbonyl compounds” (1). The solvents were divided into two groups according to their ability to isomerize compounds. The study of the keto–enol tautomerism of β-diketones and β-ketoesters in a variety of solvents using proton NMR has been utilized as a physical Figure 1. The β-dicarbonyl compounds studied in the experiment. chemistry experiment for many years (2, 3). The first reported use of NMR keto–enol equilibria determination was by Reeves (4). This technique has been described in detail in an experiment by Garland, Nibler, and Shoemaker (2). panded (i) to give an in-depth analysis of factors influencing The most commonly used β-diketone for these experi- solvent effects in tautomeric equilibria and (ii) to illustrate ments is acetylacetone (Scheme I). Use of proton NMR is a the use of molecular modeling in determining the origin of viable method for measuring this equilibrium because the a molecule’s polarity. The experiment’s original benefits of tautomeric keto–enol equilibrium is slow on the NMR time using proton NMR as a noninvasive method of evaluating scale, but enol (2a)–enol (2b) tautomerism is fast on this scale equilibrium are maintained. (5). It has been observed that acyclic β-diketones and β- Experimental Procedure ketoesters follow Meyer’s rule of a shift in the tautomeric equi- librium toward the keto tautomer with increasing solvent Observations of the solvent effects for three other 1,3- polarity (6). -

Recoverable Phospha-Michael Additions Catalyzed by a 4-N,N
molecules Article Recoverable Phospha-Michael Additions Catalyzed by a 4-N,N-Dimethylaminopyridinium Saccharinate Salt or a Fluorous Long-Chained Pyridine: Two Types of Reusable Base Catalysts Eskedar Tessema 1,†, Vijayanath Elakkat 1,† , Chiao-Fan Chiu 2,3,*, Jing-Hung Zheng 1, Ka Long Chan 1, Chia-Rui Shen 4,5, Peng Zhang 6,* and Norman Lu 1,7,* 1 Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taipei 106, Taiwan; [email protected] (E.T.); [email protected] (V.E.); [email protected] (J.-H.Z.); [email protected] (K.L.C.) 2 Department of Pediatrics, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 3 Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan 4 Department of Medical Biotechnology and Laboratory Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan; [email protected] 5 Department of Ophthalmology, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 6 Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, USA 7 Development Center for Smart Textile, National Taipei University of Technology, Taipei 106, Taiwan Citation: Tessema, E.; Elakkat, V.; * Correspondence: [email protected] (C.-F.C.); [email protected] (P.Z.); Chiu, C.-F.; Zheng, J.-H.; Chan, K.L.; [email protected] (N.L.) Shen, C.-R.; Zhang, P.; Lu, N. † These authors contributed equally to this work. Recoverable Phospha-Michael Additions Catalyzed by a Abstract: Phospha-Michael addition, which is the addition reaction of a phosphorus-based nucle- 4-N,N-Dimethylaminopyridinium ophile to an acceptor-substituted unsaturated bond, certainly represents one of the most versatile and Saccharinate Salt or a Fluorous powerful tools for the formation of P-C bonds, since many different electrophiles and P nucleophiles Long-Chained Pyridine: Two Types can be combined with each other. -

Development of the Interrupted Nazarov Cyclization of Allenyl Vinyl Ketones, with Application to the Total Synthesis of the Cyclooctane Natural Product Roseadione
Development of the Interrupted Nazarov Cyclization of Allenyl Vinyl Ketones, with Application to the Total Synthesis of the Cyclooctane Natural Product Roseadione by Vanessa M. Marx Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia May 2011 © Copyright by Vanessa M. Marx, 2011 DALHOUSIE UNIVERSITY DEPARTMENT OF CHEMISTRY The undersigned hereby certify that they have read and recommend to the Faculty of Graduate Studies for acceptance a thesis entitled “Development of the Interrupted Nazarov Cyclization of Allenyl Vinyl Ketones, with Application to the Total Synthesis of the Cyclooctane Natural Product Roseadione” by Vanessa M. Marx in partial fulfilment of the requirements for the degree of Doctor of Philosophy. Dated: May 19, 2011 Supervisor: _________________________________ Readers: _________________________________ _________________________________ _________________________________ Departmental Representative: _________________________________ ii DALHOUSIE UNIVERSITY DATE: May 19, 2011 AUTHOR: Vanessa M. Marx TITLE: Development of the Interrupted Nazarov Cyclization of Allenyl Vinyl Ketones, with Application to the Total Synthesis of the Cyclooctane Natural Product Roseadione DEPARTMENT OR SCHOOL: Department of Chemistry DEGREE: PhD CONVOCATION: October YEAR: 2011 Permission is herewith granted to Dalhousie University to circulate and to have copied for non-commercial purposes, at its discretion, the above title upon the request of individuals or institutions. I understand that my thesis will be electronically available to the public. The author reserves other publication rights, and neither the thesis nor extensive extracts from it may be printed or otherwise reproduced without the author’s written permission. The author attests that permission has been obtained for the use of any copyrighted material appearing in the thesis (other than the brief excerpts requiring only proper acknowledgement in scholarly writing), and that all such use is clearly acknowledged. -

Electron Deficient Heterocycle. Examples Abound
Pyridines Pyridines are the most commonly encountered π- electron deficient heterocycle. Examples abound. H O 4 N nifedipine 5 3 N NH2 (anti- angina) H CH3 6 2 MeO2C CO2Me N N 1 N NO2 nicotine niacin (nicitinamide) (in NADP) There are many classical syntheses of pyridines; only a few can be covered in the time available. 1. Hantzsch Synthesis Probably the conceptually simples pyridine synthesis would be a simple extension based on the Paal- Knorr pyrrole synthesis. In other words…. Paal-Knorr NH3 R5 R2 5 2 R N R OO H the shouldn't a 1,5-dicarbonyl do.... NH3 [O] 6 2 6 2 R R 6 2 R N R O O R N R H This is absolutely feasible. Furthermore, as you might expect, the 1,5-dicarbonyls are readily accessible by Michael reaction chemistry (1,4-, conjugate additions). The one- pot version of this, where the unsaturated carbonyl is prepared, the Michael reaction occurs, and the pyridine formation is accomplished, is quite common. It is called the Hantzsch synthesis. 4 O O 4 R O NH3 or O R O EtO2C CO2Et R4 NH4OAc EtO + + OEt EtO OEt ∆∆∆ H2O, O O O O OO ** R4 R4 FeCl3 EtO2C CO2Et EtO2C CO2Et ∆∆∆ H2O, N N H It’s rather forceful conditions, but the ester groups can be decarboxylated. The shown example is a symmetric case, but unsymmetrical ones can be done by doing the aldol condensation (here called a Knoevenagel) first, discretely, and then putting that compound (** ) into a separate reaction doing the subsequent steps.