22.7 Alkylation of Ester Enolate Ions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2-Adamantylacetic Acid* B
CROATICA CHEM1CA ACTA CcACAA 48 (2) I69-i78 (1976) YU ISSN 0011-1643 CCA-927 547.466:542.91 Originai Scientific Paper Synthesis of a-Amino-1-Adamantylacetic and a-Amino -2-Adamantylacetic Acid* B . Gaspert, S. Hromadko, and B. Vranesic Research Department »Piiva«, Pharmaceuticai and Chemicai Works, Zagreb, Croatia, Yugosiavia Rece ived September 29 , 1975 a-Amino-2-adamantylacetic acid (VII) was obtained when 2-adamantylcyanoacetylhydrazide (IX) was subjected to Curtius rearrangement or 2-(2-adaman;tyl)-malonamic acid (XII) to Hof mann degradation. The same amino acid was obtained when ri. -bromo-2-adamantylacetic acid (VI) was •treated with ammonia. Partial hydrolysis of diethyl adamantyl-(1)-malonate (XIV) yie1ded ethyl adamantyl-(1)-malcmate (XV) which, after treatment with thionyl chlodde and then ammonia, gave 2-(1-adamantyl) -malonamic acid ethyl ester (XVII). Hofmann degradation of 2-(1 -adamantyl)-malonamic acid ethyl ester gave a-amino-1- -adama:ntylacetic acid (XVIII). Discovery of interesting biological properties of 1-aminoadamantane hy drochloride' ·stimulated the synthesis of a large number of structurally related compounds. The synthesis of amino acids, having an adamantyl group as a part of the 2 molecule, has been reported for 3-aminoadamantane-1-carboxyHc acid , a -amino-1-adamantylacetic acid3 and recently for 2-'aminoadamantane-2-car boxylic acid4. It seemed appmpriate to us to synthetise a-amLno-2-adamantyl acetic acid5 and to elaborate a moire convenient synthesis of a-amino-1-ada ma:ntylacetic acid6, which -

Synthesis of 3-Fluorooxindole Derivatives Using Diethyl 2-Fluoromalonate
Durham E-Theses Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives HARSANYI, ANTAL How to cite: HARSANYI, ANTAL (2013) Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/6357/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk A Thesis Entitled Synthesis of 3-fluoro-oxindoles and phenyl fluoroacetic acid derivatives Submitted by Antal Harsanyi BSc (College of St. Hild and St. Bede) Department of Chemistry A Candidate for the Degree of Master of Science 2012 Declaration The work presented within this thesis was carried out at Durham University between October 2011 and August 2012. This thesis is the work of the author, except where acknowledged by reference and has not been submitted for any other degree. Part of this work has been presented at: 12th RSC Annual Fluorine Group Meeting, St Andrews, August 2012 Statement of copyright The copyright of this thesis rests with the author. -

The Development and Application of Metal-Catalyzed Processes for Organic Synthesis
The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy B.A. Chemistry Northwestern University, 2000 Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN ORGANIC CHEMISTRY at the Massachusetts Institute of Technology June 2005 © 2005 Massachusetts Institute of Technology All Rights Reserved Signature of Author: " - - 1_"' .Da-uirnt of Chemistry May 19, 2005 Certified by: Stephen L. Buchwald Camille Dreyfus Professor of Chemistry Thesis Supervisor Accepted by: Robert W. Field Chairman, Departmental Committee on Graduate Students ARCHIVES: This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: -/ Professor Gregory C. Fu: " Ch Chair Professor Stephen L. Buchwald Thesis Supervisor Professor Timothy M. Swager: , -L i \ O 2 The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy Submitted to the Department of Chemistry on May 19, 2005 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy at the Massachusetts Institute of Technology ABSTRACT Chapter 1. Copper-Catalyzed Arylation of Stabilized Carbanions A mild, general catalytic system for the synthesis of a-aryl malonates has been developed. Aryl iodides bearing a variety of functional groups can be effectively coupled to diethyl malonate in high yields using inexpensive and widely available reagents, making this a superior method to those previously described that employ copper reagents or catalysts. The functional group tolerance of the process developed makes it complementary to analogous palladium-catalyzed couplings. Importantly, a set of mild reaction conditions has been developed that minimize product decomposition, a problem that had not been addressed previously in the literature. -

Design, Synthesis, and Supramolecular Surface Chemistry Of
DESIGN, SYNTHESIS, AND SUPRAMOLECULAR SURFACE CHEMISTRY OF BI- AND TRIDENTATE SURFACE ANCHORS FOR NANOSCIENCE AND NANOBIOTECHNOLOGY A Dissertation Presented to The Graduate Faculty of the University of Akron In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy Hui Wang August, 2007 DESIGN, SYNTHESIS, AND SUPRAMOLECULAR SURFACE CHEMISTRY OF BI- AND TRIDENTATE SURFACE ANCHORS FOR NANOSCIENCE AND NANOBIOTECHNOLOGY Hui Wang Dissertation Approved: Accepted: _______________________ _______________________ Advisor Department Chair Dr. Jun Hu Dr. Kim C. Calvo _______________________ _______________________ Committee Member Dean of the College Dr. Gerald F. Koser Dr. Ronald F. Levant _______________________ _______________________ Committee Member Dean of the Graduate School Dr. David A. Modarelli Dr. George R. Newkome _______________________ _______________________ Committee Member Date Dr. Claire A. Tessier _______________________ Committee Member Dr. Robert R. Mallik ii ABSTRACT This dissertation describes the design, synthesis, and supramolecular surface chemistry of bi- and tri-dentate surface anchors for nanoscience and nanobiotechnology. Molecular electronic device candidates, based on the tridentate surface anchor 2,4,9-trithia-tricyclo[3.3.1.13,7]decane, were used to bridge two ruthenium metal clusters. These well-designed ruthenium complexes were used as nanometer-sized molecular connector/metal cluster models to investigate the surface binding characteristics of tridentate surface anchor-metal junctions. Bi-dentate surface anchors, 1,4-dimercapto-2,3-dimethyl-butane- 2,3-diol and 4,5-dimethyl-2-(4-vinyl-phenyl)-[1,3,2]dioxaborolane-4,5-dithiol, were synthesized. They were used as ligands for stabilizing gold nanoparticles by two methods: direct reduction reaction, and ligand exchange reaction with triphenylphosphine-stabilized gold nanoparticles. -

Development of Novel Peptide Nucleic Acids A
b-Dicarbonyl compounds Compounds having two carbonyl groups separated by an intervening carbon atom are called b-dicarbonyl compounds, and these compounds are highly versatile reagents for organic synthesis. O O O O C C C R C C C OR' b b b-Dicarbonyl system b-Keto ester ** The pKa for such a proton is in the range 9-11, acidic enough to be removed easily by an alkoxide base to form an enolate. O O O H O OR C C C C C.. C + HOR H enolate anion pKa = 9-11 .. .. .. .. .. .. O . O . O O . O O C C C .. C C C R C OR' R C OR' R C OR' H H H Resonance structure of the anion of a b-keto ester Synthesis of b-keto ester (Claisen condensation) O O O NaOC H 2 2 5 CH3COC2H5 CH3CC..HCOC2H5 + C2H5OH + Na (removed by distillation) Sodiumacetoacetic ester HCl O O CH3CCH2COC2H5 Ethyl acetoacetate (acetoacetic ester) (76%) O O O O (1) NaOC2H5 R CH C + H CHC R CH2C CHCOC H + C H OH 2 OC2H5 OC2H5 + 2 5 2 5 (2) H3O R R (R may also be H) b-Keto ester Mechanism Step1 .. O O . .. + . OHC H + C H OH R CHC OC2H5 .. 2 5 RC.. H C OC2H5 2 5 H .. O . RCH C OC2H5 Step 2 .. .. .. .. O . O O O . RCH2C + . RCH2C CH C OC2H5 HC C OC2H5 . C H O . OC2H5 R 2 5 .. R .. O . O .. + . OC2H5 RCH2C CH C OC2H5 .. R Step 3 .. .. O . O H O O . -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -
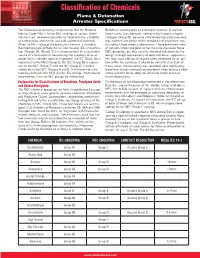
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Regioselective Addition of 1,3-Dicarbonyl Dianion to N-Sulfonyl Aldimines: an Expedient Route to N-Sulfonyl Piperidines and N-Sulfonyl Azetidines
Tetrahedron 63 (2007) 4779–4787 Regioselective addition of 1,3-dicarbonyl dianion to N-sulfonyl aldimines: an expedient route to N-sulfonyl piperidines and N-sulfonyl azetidines Manas K. Ghorai,* Amit Kumar and Sandipan Halder Department of Chemistry, Indian Institute of Technology, Kanpur 208 016, India Received 26 June 2006; revised 23 February 2007; accepted 15 March 2007 Available online 18 March 2007 Abstract—A simple route for the synthesis of d-amino-b-keto esters and d-amino-b-diketones is reported. This involves regioselective addition of 1,3-dianions derived from ethyl acetoacetate and acetyl acetone to N-sulfonyl aldimines. The d-amino-b-keto ester derivatives were further converted into the corresponding N-sulfonyl piperidines and N-sulfonyl azetidines. Ó 2007 Elsevier Ltd. All rights reserved. 1. Introduction In continuation of our research on enolate chemistry and also supported by the related literature on the reactivity of 1,3-di- 1,3-Dicarbonyl dianions have been used extensively in or- carbonyl dianions, it was envisaged that dianions derived ganic synthesis for building ring systems. The anion on the from suitable precursors could be regioselectively added to terminal carbon of the dianion can be regioselectively reacted aldimines to give an easy access to d-amino-b-keto esters18 with 1 equiv of an electrophile resulting in an enolate ion, and their diketone analogues. These compounds could easily which can be trapped by the addition of a second electrophile be converted into various piperidine and azetidine deriva- -

Ethyl Acetoacetate (Acetoacetic Ester) (B) Diethyl Molonote (Molonic Ester) the Structure of (A) and (B) Are As
PRESENTATION OF ENOLATES Dr. Susmita Bajpai Department of Chemistry B.N.D. College, Kanpur ENOLATES The class of compounds which contain a methylene group (–CH2–) directly bonded to the electron withdrawing groups such as –COCH3, –COOC2H5, –CN, are called active methylene compounds. This is so because the –CH2 group in them is acidic and reactive. The two examples are (a) Ethyl acetoacetate (Acetoacetic ester) (b) Diethyl molonote (Molonic ester) The structure of (a) and (b) are as This reaction is known as claisen condensation Ethyl acetoacetate (CH3COOCH2COOC2H5) • It's IUPAC name is ethyl 3-oxobutanoate • Preparation: Ethyl acetoacetate is prepared by heating ethyl acetate with sodium ethoxide in ethanol, followed by acidification. Claisen condensation • It is a condensation reaction in which, two ester molecules condensed to form an alcohol and a b-Keto ester. Therefore ethyl acetoacetate is a b-Keto ester. • Mechanism: The mechanism involves three steps: + • Step-I :- First sodium ethoxide (C2H5O–Na ) breaks into ethoxide ion and sodium ion. This ethoxide ion attacks ethyl acetoacetate to give ethyl alcohol and ester anion. Step -II: Ester anion attacks the carbonyl group of a second molecules of ethyl acetate. • Step-III: Ethoxide ion is eliminated Properties • It is a colourless pleasant smelling liquid • b.p. - 180.4oC • It is sparingly soluble in water but freely so in organic solvent. • It is neutral to litmus. Chemical properties • It is a tautomeric mixture of Keto and enol forms. Therefore it gives the reaction of the various functional groups present in the two forms. Acidity of methylene hydrogen (Formation of salt) • In ethyl acetoacetate methylene group (–CH2–) flank by two carbonyl group. -
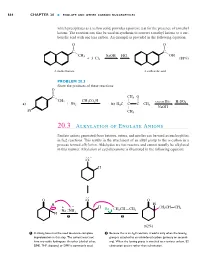
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Development of New Domino Reactions of Alkylidene Meldrum's
Development of New Domino Reactions of Alkylidene Meldrum’s Acids Involving Friedel-Crafts Chemistry and Catalytic Conjugate Allylation of Alkylidene Meldrum’s Acids by Aaron Michael Dumas A thesis presented to the University of Waterloo in fulfilment of the thesis requirement for the degree of Doctor of Philosophy in Chemistry Waterloo, Ontario, Canada, 2009 © Aaron Michael Dumas 2009 I hereby declare that I am the sole author of this thesis. This is a true copy of my thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Alkylidene Meldrum’s acids are very reactive acceptors in conjugate additions, and are known to be significantly more electrophilic than other α,β-unsaturated carbonyl electrophiles. They also offer advantages in terms of ease of preparation, purification and storage. Despite this, they are relatively underused in organic synthesis, and have been treated as something of a curiousity in the literature. The goal of my research was to demonstrate the utility of these molecules in new reactions that are not readily available to other electrophiles. To facilitate this work, new conditions for the Knoevenagel condensation of aldehydes with Meldrum’s acid were developed. This allowed access to a broader range of monosubstituted alkylidenes than was previously possible from any single method. In a reaction that exploits the acylating ability of Meldrum’s acid, a domino addition of phenols to alkylidene Meldrum’s acids was developed. Here, Yb(OTf)3 catalyzed the addition of a phenol to the alkylidene as well as acylation through activation of the electrophile.