The Nondecussating Pathway of the Dentatorubrothalamic Tract in Humans: Human Connectome-Based Tractographic Study and Microdissection Validation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -

A Network of Genetic Repression and Derepression Specifies Projection
A network of genetic repression and derepression INAUGURAL ARTICLE specifies projection fates in the developing neocortex Karpagam Srinivasana,1, Dino P. Leonea, Rosalie K. Batesona, Gergana Dobrevab, Yoshinori Kohwic, Terumi Kohwi-Shigematsuc, Rudolf Grosschedlb, and Susan K. McConnella,2 aDepartment of Biology, Stanford University, Stanford, CA 94305; bMax-Planck Institute of Immunobiology and Epigenetics, 79108 Freiburg, Germany; and cLawrence Berkeley National Laboratory, Berkeley, CA 94720 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2011. Contributed by Susan K. McConnell, September 28, 2012 (sent for review August 24, 2012) Neurons within each layer in the mammalian cortex have stereotypic which results in a suppression of corticothalamic and callosal projections. Four genes—Fezf2, Ctip2, Tbr1, and Satb2—regulate fates, respectively, and axon extension along the corticospinal these projection identities. These genes also interact with each tract (CST). Fezf2 mutant neurons fail to repress Satb2 and Tbr1, other, and it is unclear how these interactions shape the final pro- thus their axons cross the CC and/or innervate the thalamus jection identity. Here we show, by generating double mutants of inappropriately. Fezf2, Ctip2, and Satb2, that cortical neurons deploy a complex In callosal projection neurons, Satb2 represses the expression Ctip2 Bhlhb5 genetic switch that uses mutual repression to produce subcortical of and , leading to a repression of subcerebral fates. Satb2 Tbr1 or callosal projections. We discovered that Tbr1, EphA4, and Unc5H3 Interestingly, we found that promotes expression in fi upper layer callosal neurons, and Tbr1 expression in these neu- are critical downstream targets of Satb2 in callosal fate speci ca- fi tion. -

The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2
667 The Cerebellum in Sagittal Plane-Anatomic-MR Correlation: 2. The Cerebellar Hemispheres Gary A. Press 1 Thin (5-mm) sagittal high-field (1 .5-T) MR images of the cerebellar hemispheres James Murakami2 display (1) the superior, middle, and inferior cerebellar peduncles; (2) the primary white Eric Courchesne2 matter branches to the hemispheric lobules including the central, anterior, and posterior Dean P. Berthoty1 quadrangular, superior and inferior semilunar, gracile, biventer, tonsil, and flocculus; Marjorie Grafe3 and (3) several finer secondary white-matter branches to individual folia within the lobules. Surface features of the hemispheres including the deeper fissures (e.g., hori Clayton A. Wiley3 1 zontal, posterolateral, inferior posterior, and inferior anterior) and shallower sulci are John R. Hesselink best delineated on T1-weighted (short TRfshort TE) and T2-weighted (long TR/Iong TE) sequences, which provide greatest contrast between CSF and parenchyma. Correlation of MR studies of three brain specimens and 11 normal volunteers with microtome sections of the anatomic specimens provides criteria for identifying confidently these structures on routine clinical MR. MR should be useful in identifying, localizing, and quantifying cerebellar disease in patients with clinical deficits. The major anatomic structures of the cerebellar vermis are described in a companion article [1). This communication discusses the topographic relationships of the cerebellar hemispheres as seen in the sagittal plane and correlates microtome sections with MR images. Materials, Subjects, and Methods The preparation of the anatomic specimens, MR equipment, specimen and normal volunteer scanning protocols, methods of identifying specific anatomic structures, and system of This article appears in the JulyI August 1989 issue of AJNR and the October 1989 issue of anatomic nomenclature are described in our companion article [1]. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -

Bilateral Cerebellar Dysfunctions in a Unilateral Meso-Diencephalic Lesion
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.44.4.361 on 1 April 1981. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1981, 44, 361-363 Short report Bilateral cerebellar dysfunctions in a unilateral meso-diencephalic lesion D VON CRAMON From the Max-Planck-Institute for Psychiatry, Munich, Germany SUMMARY The clinical syndrome of a 65-year-old patient with a slit-shaped right-sided meso- diencephalic lesion was analysed. A cerebellar syndrome with limb-kinetic ataxia, intention tremor and hypotonicity in all extremities as well as ataxic dysarthria was found. The disruption of the two cerebello-(rubro)-thalamic pathways probably explained the signs of bilateral cere- bellar dysfunction. The uncrossed ascending limb of the right, and the crossed one of the left brachium conjunctivum may have been damaged by the unilateral lesion extending between caudal midbrain and dorsal thalamus. Protected by copyright. Most of the fibres which constitute the superior general hospital where neurological examination cerebellar peduncle leave the cerebellum and showed bilateral miosis, convergent strabism, vertical originate in cells of the dentate nucleus but also gaze paresis on upward gaze with gaze-paretic nystag- arise from neurons of the globose and emboli- mus, flaccid sensori-motor hemiparesis with increased stretch reflexes and Babinski sign on the left side, forme nuclei. The crossed ascending fibres of the and dysmetric movements of the right upper extremity. brachia conjunctiva constitute the major outflow The CT scan showed an acute haemorrhage in the from the cerebellum, they form the cerebello- right mesodiencephalic area. On 19 February 1979 (rubro)-thalamic and dentato-thalamic tracts.' the patient was admitted to our department. -

Anatomy of Cerebellum Rajasekhar Sajja Srinivasa Siva Naga
Chapter Anatomy of Cerebellum Rajasekhar Sajja Srinivasa Siva Naga Abstract The cerebellum receives inputs from spinal cord, cerebrum, brainstem, and sensory systems of the body and controls the motor system of the body. The Cerebellum harmonizes the voluntary motor activities such as maintenance of posture and equilibrium, and coordination of voluntary muscular activity including learning of the motor behaviours. Cerebellum occupies posterior cranial fossa, and it is relatively a small part of the brain. It weighs about one tenth of the total brain. Cerebellar lesions do not cause motor or cognitive impairment. However, they cause slowing of movements, tremors, lack of equilibrium/balance. Complex motor action becomes shaky and faltering. Keywords: Cerebellum, Spinocerebellar ataxia, Cortex, Medulla, Peduncles, Nuclei 1. Introduction The Cerebellum is the largest part of the hindbrain and develops from the alar plates (rhombic lips) of the metencephalon. It lies between the temporal and occipital lobes of cerebrum and the brainstem in the posterior cranial fossa. It is attached to the posterior surface of the brainstem by three large white fibre bundles. It is attached to the midbrain by superior cerebel- lar peduncle, pons by middle cerebellar peduncle, and medulla by inferior cerebellar peduncle. Cerebellum is concerned with three primary functions: a) coordination of voluntary motor functions of the body initiated by the cerebral cortex at an uncon- scious level, b) maintenance of balance, and posture, c) Maintenance of muscle tone. It receives and integrates the sensory inputs from the cerebrum and the spinal cord necessary for a planning and smooth coordination of the movements [1]. Cerebellar lesions result in irregular and uncoordinated, awkward intentional muscle movements. -

Cerebellum and Inferior Olive
Cerebellum and Inferior Olivary Nucleus Spinocerebellum • Somatotopically organised (vermis controls axial musculature; intermediate hemisphere controls limb musculature) • Control of body musculature • Inputs… Vermis receives somatosensory information (mainly from the trunk) via the spinocerebellar tracts and from the spinal nucleus of V. It receives a direct projection from the primary sensory neurons of the vestibular labyrinth, and also visual and auditory input from brain stem nuclei. • Intermediate hemisphere receives somatosensory information (mainly from the limbs) via the spinocerebellar tracts (the dorsal spinocerebellar tract, from Clarke’s nucleus of the lower limb, and the cuneocerebellar tract, from the accessory cu- neate nucleus of the upper limb, carry information from muscle spindle afferents; both enter via the ipsilateral inferior cerebellar peduncle). • An internal feedback signal arrives via the ventral spinocerebellar tract (lower limb) and rostral spinocerebellar tract (upper limb). (Ventral s.t. decussates in the spinal cord and enters via the superior cerebellar peduncle, but some fibres re-cross in the cerebellum; rostral s.t. is an ipsilateral pathway and enters via sup. & inf. cerebellar peduncles.) • Outputs to fastigial nucleus, which projects to the medial descending systems: (1) reticulospinal tract [? n. reticularis teg- menti pontis and prepositus hypoglossi?]; (2) vestibulospinal tract [lateral and descending vestibular nn.]; and (3) an as- cending projection to VL thalamus [Å cells of origin of the ventral corticospinal tract]; (4) reticular grey of the midbrain [=periaqueductal?]; (5) inferior olive [medial accessory, MAO]. • … and interposed nuclei, which project to the lateral descending systems: (1) magnocellular portion of red nucleus [Å ru- brospinal tract]; (2) VL thalamus [Å motor cx which gives rise to lateral corticospinal tract]; (3) reticular nucleus of the pontine tegmentum; (4) inferior olive [dorsal accessory, DAO]; (5) spinal cord intermediate grey. -
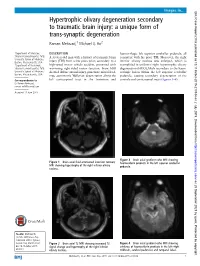
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

ON-LINE FIG 1. Selected Images of the Caudal Midbrain (Upper Row
ON-LINE FIG 1. Selected images of the caudal midbrain (upper row) and middle pons (lower row) from 4 of 13 total postmortem brains illustrate excellent anatomic contrast reproducibility across individual datasets. Subtle variations are present. Note differences in the shape of cerebral peduncles (24), decussation of superior cerebellar peduncles (25), and spinothalamic tract (12) in the midbrain of subject D (top right). These can be attributed to individual anatomic variation, some mild distortion of the brain stem during procurement at postmortem examination, and/or differences in the axial imaging plane not easily discernable during its prescription parallel to the anterior/posterior commissure plane. The numbers in parentheses in the on-line legends refer to structures in the On-line Table. AJNR Am J Neuroradiol ●:●●2019 www.ajnr.org E1 ON-LINE FIG 3. Demonstration of the dentatorubrothalamic tract within the superior cerebellar peduncle (asterisk) and rostral brain stem. A, Axial caudal midbrain image angled 10° anterosuperior to posteroinferior relative to the ACPC plane demonstrates the tract traveling the midbrain to reach the decussation (25). B, Coronal oblique image that is perpendicular to the long axis of the hippocam- pus (structure not shown) at the level of the ventral superior cerebel- lar decussation shows a component of the dentatorubrothalamic tract arising from the cerebellar dentate nucleus (63), ascending via the superior cerebellar peduncle to the decussation (25), and then enveloping the contralateral red nucleus (3). C, Parasagittal image shows the relatively long anteroposterior dimension of this tract, which becomes less compact and distinct as it ascends toward the thalamus. ON-LINE FIG 2. -

Calcification of the Corpus Striatum and Dentate Nuclei Occurring in a Family by J
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.14.4.253 on 1 November 1951. Downloaded from J. Neurol. Neurosurg. Pgchiat., 1951, 14, 253. CALCIFICATION OF THE CORPUS STRIATUM AND DENTATE NUCLEI OCCURRING IN A FAMILY BY J. FOLEY Fronm the Neurological Department, St. George's Hospital, London Dense symmetrical calcification of the corpus no previous illnesses of note, and on direct questioning striatum is rare, but of some general interest for there was no history of tetany or thyroid disorder. two reasons. First, in a significant number of the There was no family history of mental disturbance, reported cases this apparently degenerative condi- epilepsy, or endocrine abnormality. The patient had had seven children. The first died in convulsion at three tion is closely linked with a metabolic disorder, months, but the remaining six are well. Four years hypoparathyroidism, which may be idiopathic, before admission it was noticed that the patient was parathyroprivic, or of the variety in which the becoming slower in her movements, and that her memory parathyroid glands are histologically normal but was failing. At about the same time she began to have guest. Protected by copyright. the body is unable to respond to parathormone brief attacks of vertigo, in one of which she fell forwards, (Albright's pseudohypoparathyroidism). Secondly, cutting her head and leg; none of these attacks was in no other degenerative disease limited to a neuro- associated with loss of consciousness. One year before physiological system can the process be visualized admission her gait became unsteady, and after a further radiologically. In contrast with the rare gross eight months her legs were so weak and unsteady that under she was unable to climb stairs without assistance.