Tropicamide Tropicamide Eye Drops
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
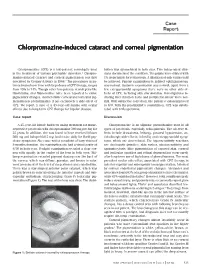
Chlorpromazine-Induced Cataract and Corneal Pigmentation
Case Report Chlorpromazine-induced cataract and corneal pigmentation Chlorpromazine (CPZ) is a low-potency neuroleptic used bution was symmetrical in both eyes. Two independent clini- in the treatment of various psychiatric disorders.1 Chlorpro- cians documented the condition. The pupils were dilated with mazine-induced cataract and corneal pigmentation was first 1% tropicamide for retinoscopy. A dilatation of only 4 mm could described by Greiner & Berry in 1964.2 The prevalence in pa- be achieved. Fundus examination by indirect ophthalmoscopy tients treated over time with large doses of CPZ therapy, ranges was normal. Systemic examination was normal; apart from a from 15% to 74%. Though other low-potency neuroleptics like few extrapyramidal symptoms there were no other side-ef- thioridazine and fluphenazine have been reported to cause fects of CPZ, including skin discoloration. Investigations in- pigmentary changes, characteristic corneal and lenticular pig- cluding liver function tests and peripheral smear were nor- mentation is predominantly if not exclusively a side-effect of mal. With subjective correction, the patient’s vision improved CPZ. We report a case of a 45-year-old female with ocular to 6/9. With the psychiatrist’s consultation, CPZ was substi- effects due to long-term CPZ therapy for bipolar disease. tuted with trifluoperazine. Case report Discussion A 45-year-old female had been taking treatment for manic- Chlorpromazine is an aliphatic phenothiazine used in all depressive psychosis with chlorpromazine 300 mg per day for types of psychosis, especially schizophrenia. The adverse ef- 25 years. In addition, she was found to have received lithium fects include drowsiness, lethargy, postural hypotension, an- 300 mg and haloperidol 5 mg, both twice daily for florid psy- ticholinergic side-effects, infertility and extrapyramidal symp- chotic symptoms. -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

Acepromazine and Chlorpromazine As Pharmaceutical-Grade Alternatives to Chlorprothixene for Pupillary Light Reflex Imaging in Mice
Journal of the American Association for Laboratory Animal Science Vol 59, No 2 Copyright 2020 March 2020 by the American Association for Laboratory Animal Science Pages 197–203 Acepromazine and Chlorpromazine as Pharmaceutical-grade Alternatives to Chlorprothixene for Pupillary Light Reflex Imaging in Mice Samantha S Eckley,1 Jason S Villano,1 Nora S Kuo,1 and Kwoon Y Wong2,* Studies of visual responses in isoflurane-anesthetized mice often use the sedative chlorprothixene to decrease the amount of isoflurane used because excessive isoflurane could adversely affect light-evoked responses. However, data are not available to justify the use of this nonpharmaceutical-grade chemical. The current study tested whether pharmaceutical-grade sedatives would be appropriate alternatives for imaging pupillary light reflexes. Male 15-wk-old mice were injected intraperitoneally with 1 mg/kg chlorprothixene, 5 mg/kg acepromazine, 10 mg/kg chlorpromazine, or saline. After anesthetic induction, anes- thesia maintenance used 0.5% and 1% isoflurane for sedative- and saline-injected mice, respectively. A photostimulus (16.0 log photons cm−2 s−1; 470 nm) was presented to the right eye for 20 min, during which the left eye was imaged for consensual pupillary constriction and involuntary pupil drift. Time to immobilization, loss of righting reflex, physiologic parameters, gain of righting reflex, and degree of recovery were assessed also. The sedative groups were statistically indistinguishable for all measures. By contrast, pupillary drift occurred far more often in saline-treated mice than in the sedative groups. Fur- thermore, saline-treated mice took longer to reach maximal pupil constriction than all sedative groups and had lower heart rates compared with chlorpromazine- and chlorprothixene-sedated mice. -

208151Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 208151Orig1s000 SUMMARY REVIEW NDA 208151, ISOPTO Atropine (atropine sulfate ophthalmic solution) 1% Cycloplegia, Mydriasis, Penalization of the healthy eye in the treatment of amblyopia Division Director Summary Review Material Reviewed/Consulted OND Action Package, including: Names of discipline reviewers Medical Officer Review Wiley Chambers, William Boyd 9/13/2016 CDTL Review William Boyd, Wiley Chambers 11/29/2016 Statistical Review Abel Eshete, Yan Wang 11/8/2016 Pharmacology Toxicology Review Aaron Ruhland, Lori Kotch 11/7/2016 CMC Review Haripada Sanker, Milton Sloan, Maotang Zhou, Denise Miller, Vidya Pai, Om Anand, Erin Andrews, Chunchun Zhang, Paul Perdue 10/12/2016, 11/10/2016 Clinical Pharmacology Review Abhay Joshi, Philip Colangelo 11/9/2016 OPDP/DPDP Carrie Newcomer 11/7/2016 OSI/DGCPC N/A Proprietary Name Michelle Rutledge, Yelena Maslov, Lubna Merchant 6/1/2016 Conditionally acceptable letter Todd Bridges 6/2/2016 OSE/DMEPA Michelle Rutledge, Yelena Maslov 6/8/2016 OSE/DDRE N/A OSE/DRISK N/A Project Manager Michael Puglisi OND=Office of New Drugs CDTL=Cross-Discipline Team Leader OSI/DGCPC=Office of Scientific Investigations/Division of Good Clinical Practice Compliance OPDP/DPDP=Office of Prescription Drug Promotion/Division of Prescription Drug Promotion OSE= Office of Surveillance and Epidemiology DMEPA=Division of Medication Error Prevention and Analysis DDRE= Division of Drug Risk Evaluation DRISK=Division of Risk Management Page 2 of 24 Reference ID: 4021108 NDA 208151, ISOPTO Atropine (atropine sulfate ophthalmic solution) 1% Cycloplegia, Mydriasis, Penalization of the healthy eye in the treatment of amblyopia Division Director Summary Review Signatory Authority Review Template 1. -

World Health Organization Model List of Essential Medicines, 21St List, 2019
World Health Organizatio n Model List of Essential Medicines 21st List 2019 World Health Organizatio n Model List of Essential Medicines 21st List 2019 WHO/MVP/EMP/IAU/2019.06 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. -

Tropicamide 2018 Newborn Use Only
Tropicamide 2018 Newborn Use Only Alert Watch for apnoeas and abdominal distension following administration. Lower concentration solutions and regimens minimising number of additional drops are recommended. Indication Induction of mydriasis and cycloplegia for diagnostic and therapeutic ophthalmic procedures. Action Anticholinergic drug that produces pupillary dilatation by inhibiting the sphincter pupillae muscle and paralysis of accommodation. Drug Type Antimuscarinic. Trade Name Minims Tropicamide Eye Drops Mydriacyl Eye drops Presentation Minims Tropicamide Eye Drops 0.5%, 1.0% solution. Mydriacyl Eye drops 0.5%, 1.0% 15 mL (multidose—excipients benzalkonium chloride 0.01%, sodium chloride, disodium edetate, hydrochloric acid and/or sodium hydroxide, purified water). Dosage/Interval Use in combination with phenylephrine 2.5% with or without cyclopentolate 0.5%. REGIMEN 1: Phenylephrine 2.5% + cyclopentolate 0.5% + tropicamide 0.5% eye drops [1-4]. Instil one drop of each agent (5 minutes apart) into each eye 60 minutes prior to examination. Repeat if pupillary dilatation inadequate. Perform examination 60 to 120 minutes after instillation. REGIMEN 2: Phenylephrine 2.5% + tropicamide 0.5% eye drops [5-7]. Instil one drop of each agent (5 minutes apart) into each eye 60 minutes prior to examination. Repeat if pupillary dilatation inadequate. Perform examination 60 to 120 minutes after instillation. Dark irides may require additional drops. Maximum daily dose REGIMEN 1: 3 drops of each agent. REGIMEN 2: 4 drops of each agent. Route Topical instillation into the eyes from the container or use a microdrop (5–7 microL) cannula. Preparation/Dilution Administration Apply pressure to the lacrimal sac during and for 60 seconds after instillation of eye drop to minimise systemic absorption. -

Maintenance Drug List Below Is Not Comprehensive and Is Subject to Change Without Prior Notification
Maintenance Drugs Maintenance drugs are used on a continuing basis for the treatment of chronic conditions. Select health plans require that pharmacy benefit maintenance drugs are filled at Kaiser Permanente Washington Mail Order or Kaiser Permanente Clinic Pharmacies for ongoing health plan coverage. Please consult your Evidence of Coverage or call Member Services at 1-888-901-4636 if you have questions about your drug coverage. Non-maintenance drugs are generally used for episodic treatment, are considered narrow therapeutic index by Kaiser Permanente Washington, or are schedule 2 controlled substances. Non-maintenance products include: opioids, opioid reversal agents, opioid dependence agents, benzodiazepines, skeletal muscle relaxants, schedule 2 stimulants, vaccines, oral antibiotics, antiemetics, cough/cold/allergy products, emergency contraception, epinephrine pens, bowel preparations, smoking cessation, prednisone, enoxaparin, cyclosporine, digoxin, dofetilide, everolimus, lithium, mycophenolate, sirolimus, sotalol, tacrolimus, theophylline, and warfarin. The maintenance drug list below is not comprehensive and is subject to change without prior notification. Coverage is provided by Kaiser Permanente Health Plan of Washington and Kaiser Permanente Health Plan of Washington Options, Inc. MAINTENANCE DRUG LIST: ADHD/ANTI-NARCOLEPSY AGENTS DICLOFENAC NALFON ARMODAFINIL DICLOFENAC POTASSIUM NAPRELAN ATOMOXETINE DICLOFENAC SODIUM DR NAPROSYN CLONIDINE HCL ER DICLOFENAC SODIUM ER NAPROXEN GUANFACINE ER DICLOFENAC SODIUM/MISOPROSTOL NAPROXEN -

Anisocoria in a Patient with Acute Bronchiolitis Akut Bronşiyolitli Bir Hastada Anizokori
Case Report / Olgu Sunumu DOI: 10.5578/ced.201806 • J Pediatr Inf 2018; 12(1): e32-e34 Anisocoria in a Patient with Acute Bronchiolitis Akut Bronşiyolitli Bir Hastada Anizokori Neslihan Kara1, Seda Çelik1, Gözde Gürpınar1, Nazan Dalgıç2, İhsan Kafadar3 1 Department of Pediatrics, Sisli Hamidiye Etfal Training and Research Hospital, Istanbul, Turkey 2 Department of Pediatric Infectious Diseases, Sisli Etfal Hamidiye Training and Research Hospital, Istanbul, Turkey 3 Department of Pediatric Neurology, Sisli Hamidiye Etfal Training and Research Hospital, Istanbul, Turkey Abstract Özet The term anisocoria refers to pupils that are of different sizes. The causes Anizokori, her iki pupillanın birbirinden farklı büyüklüklerde olması de- of anisocoria include physiological anisocoria, pharmacological anisoco- mektir. Anizokori nedenleri arasında fizyolojik anizokori, farmakolojik ria, cerebral neoplasms, and space occupying lesions such as aneurysms, anizokori, serebral neoplazi ve anevrizma gibi yer kaplayan oluşumlar and intracranial hemorrhage. The aim of the current manuscript is to ve intrakranial kanamalar bulunmaktadır. Burada akut bronşiyolit nede- present a 7-month old male case of sudden onset anisocoria who was niyle takip ettiğimiz, salbutamol ve ipratropium bromür tedavisi alan 7 on follow-up due to acute bronchiolitis and was receiving therapy with aylık erkek hastada ani gelişen anizokoriyi sunmayı amaçladık. salbutamol and ipratropium bromide. Anahtar Kelimeler: Anizokori, akut bronşiyolit, ipratropium bromür, Keywords: Anisocoria, acute bronchiolitis, ipratropium bromide, salbutamol salbutamol Introduction The causes of an abnormally small pupil include iridocy- clitis, previous eye surgery, pseudoexfoliation syndrome or Anisocoria can be the result of physiological factors but may pharmacological constriction caused by topical agents such as also occur in life-threatening conditions. -

Pupil Dilatation with Tropicamide. the Effects on Acuity, Accommodation and Refraction
Eye (1989) 3, 845-848 Pupil Dilatation with Tropicamide. The Effects on Acuity, Accommodation and Refraction D. M. I. MONTGOMERY! and C. J. MACEWANZ Glasgow and Dundee. Summary The effect of pupil dilatation with tropicamide 1 % on visual acuity and accommo dation was assessed in 100 eyes of 52 consecutive patients attending the general ophthalmic outpatient clinic. Snellen visual acuity remained unchanged in 55 eyes and deteriorated by one line in 41 eyes. The remaining four eyes deteriorated by two lines. Tropicamide gave rise to a reduction in the amplitude of accommodation which tended to vary inversely with the age of the patient. However, all patients who wore reading glasses for presbyopia were still able to read when their pupils had been dilated. Many drugs are used routinely in ophthalmic acuity and accommodation in such a practice to dilate the pupil for adequate exam population. ination of the ocular fundus. Selection may be based on the speed of onset and duration of action of the drug as well as on the presence Materials and methods and severity of any unwanted effects such as Tropicamide 1 % was used to dilate the pupils cycloplegia. Tropicamide is a drug often of 100 eyes of 52 consecutive patients who favoured because of its short duration of required fundoscopy during attendance at the action and relatively greater mydriatic than general ophthalmology outpatient clinic. The cycloplegic effect. mean patient age was 38 years (range 6-84 Concern is often expressed with regard to years). Prior to dilatation retinoscopy was adverse effects of pupil dilatation on vision carried out and the uncorrected and best cor which might affect patients' ability to travel rected visual acuities were recorded using a home safely or to return to work. -

Unilateral Mydriasis in a Child with A
Case report BMJ Case Rep: first published as 10.1136/bcr-2020-237257 on 22 December 2020. Downloaded from Unilateral mydriasis in a child with a ventriculoperitoneal shunt for obstructive hydrocephalus: a diagnostic dilemma Monidipa Banerjee, Eiman Haj Ahmed, Kathryn Foster, Arundoss Gangadharan Department of Paediatrics, SUMMARY glucose remained stable throughout the stay. Ysbyty Gwynedd, Bangor, UK There are several causes for sudden onset unilateral Blood gases were also stable with a peak lactate mydriasis, however impending transtentorial uncal of 2.6 mmol/L during salbutamol infusion. The Correspondence to herniation needs to be ruled out. This unique case theophylline levels were within the therapeutic Dr Arundoss Gangadharan; range during aminophylline infusion. A nasopha- Drarundoss@ yahoo. com highlights an uncommon adverse response to a common mode of treatment that leads to a diagnostic dilemma. A ryngeal aspirate was positive for respiratory syncy- tial virus. Chest X- ray showed minimal opacity at Accepted 30 November 2020 3-year -old boy with a ventriculoperitoneal (VP) shunt for an obstructive hydrocephalus presented with an acute left middle zone with hyperinflated lung fields. respiratory distress. He developed unilateral mydriasis with absent light reflex during treatment with nebulisers. DIFFERENTIAL DIAGNOSIS An urgent CT scan of the brain did not show any new Although he was treated for worsening acute viral- intracranial abnormality. A case of pharmacological induced wheeze, blocked VP shunt with increasing anisocoria was diagnosed that resolved completely intracranial pressure and risk of uncal herniation within 24 hours of discontinuation of ipratropium needed ruling out. bromide. Although ipratropium-induced anisocoria has been reported in children, but to our knowledge none in a child with VP shunt for hydrocephalus. -

Topical Tropicamide Induced Delirium and Psychosis: Case Report
RELATO DE CASO Topical tropicamide induced delirium and psychosis: case report Delírio e psicose induzidos por tropicamida tópica: relato de caso Renato P. Munhoz1, Mariana M. Moscovich1, Luciane Filla1, Maria Cecília Beltrame Carneiro2 ABSTRACT Acute psychosis and confusional states are known complications of treatment with anticholiner- gic agents in the elderly. We report an 87-year-old female patient presenting with acute neuro- behavioral abnormalities requiring hospitalization immediately after starting treatment for open- Keywords angle glaucoma with the topic cycloplegic muscarinic receptor blocker tropicamide. Case-effect Tropicamide, psychosis, relationship was confirmed. The authors make a review of the literature trying to identify the clinical cycloplegic, anticholinergic. manifestations and risk factors for this complication. RESUMO Tratamento com drogas anticolinérgicas é uma causa conhecida de alterações agudas do estado mental em idosos. Relata-se o caso de uma paciente de 87 anos de idade com alte- rações comportamentais agudas, que necessita de internamento imediatamente após início Palavras-chave de terapia para glaucoma de ângulo aberto com tropicamida, um agente cicloplégico blo- Tropicamida, queador de receptor muscarínico. A relação causa-efeito foi confirmada depois de a droga ter psicose, cicloplégico, sido reiniciada durante o internamento. É apresentada uma revisão da literatura delineando anticolinérgico. as manifestações clínicas mais comuns e fatores de risco para essa complicação. INTRODUCTION Although atropine is still widely used, the delay to onset of cycloplegia, the long time required for functional recovery, Acute psychosis and confusional states are known complica- and the potential for serious side effects have led to use of tions of treatment with anticholinergic agents (AChA) in the shorter acting agents3,4. -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration.