Maintenance Drug List Below Is Not Comprehensive and Is Subject to Change Without Prior Notification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity
molecules Review Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity Babiker M. El-Haj 1,* and Samrein B.M. Ahmed 2 1 Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fufairah 00971, UAE 2 College of Medicine, Sharjah Institute for Medical Research, University of Sharjah, Sharjah 00971, UAE; [email protected] * Correspondence: [email protected] Received: 6 February 2020; Accepted: 7 April 2020; Published: 22 April 2020 Abstract: Alkyl moieties—open chain or cyclic, linear, or branched—are common in drug molecules. The hydrophobicity of alkyl moieties in drug molecules is modified by metabolic hydroxy functionalization via free-radical intermediates to give primary, secondary, or tertiary alcohols depending on the class of the substrate carbon. The hydroxymethyl groups resulting from the functionalization of methyl groups are mostly oxidized further to carboxyl groups to give carboxy metabolites. As observed from the surveyed cases in this review, hydroxy functionalization leads to loss, attenuation, or retention of pharmacologic activity with respect to the parent drug. On the other hand, carboxy functionalization leads to a loss of activity with the exception of only a few cases in which activity is retained. The exceptions are those groups in which the carboxy functionalization occurs at a position distant from a well-defined primary pharmacophore. Some hydroxy metabolites, which are equiactive with their parent drugs, have been developed into ester prodrugs while carboxy metabolites, which are equiactive to their parent drugs, have been developed into drugs as per se. -

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Diabetes Medications: Oral Medications
Diabetes Medications: Oral Medications Medication Types 1. Biguanides 2. Sulfonylureas 3. Thiazolidinediones (TZDs) 4. Alpha-Glucosidase Inhibitors 5. D-Phenylalanine Meglitinides 6. SGLT-2 inhibitors 7. DPP-4 inhibitors 8. Combination Oral Medications 1. Biguanides This works by lowering blood glucose by reducing the amount of glucose produced by the liver and helping the body respond better to the insulin made in the pancreas Metformin can be used with diet and exercise or with other agents, diet, and exercise. Types of Biguanides: • Metformin (Glucophage) 500mg/1000mg • Metformin (Glucophage XR) 500mg/1000mg • Fortamet (extended release) 500mg/1000mg • Riomet (oral solution) 500mg/5ml Side Effects: • Cramping • Gas • Diarrhea • Taking the pill before meals may decrease stomach upset 2. Sulfonylureas Sulfonylureas stimulate the pancreas to produce insulin and cause the body to respond better to the insulin it does produce. Sulfonylureas can be used alone or in combination with other medications. Types of Sulfonylureas: • Glimepiride (Amaryl) • Glipizide (Glucotrol, Glucotrol XL) • Glyburide (Diabeta, Micronase) • Glyburide, micronized (Glynase) • Tolbutamide (Orinase) 1st generation • Tolazamide (Tolinase) 1st generation • Acetohexamide (Dymelor) 1st generation • Chlorpropamide (Diabinese) 1st generation Side Effects: • Hypoglycemia • Upset stomach • Weight gain • Skin rash 3. Thiazolidinediones (TZDs) TZDs primarily reduce insulin resistance by improving target cell response (sensitivity) to insulin. They also can decrease glucose output from the liver and increase glucose disposal in the skeletal muscles. Types of TZDs: • Pioglitazione (Actos) 15-45 mg Actos may be taken with or without food • Avandia—off the market Side Effects: • Jaundice • Nausea and vomiting • Stomach pain • Dark urine • Swelling • These medicines are generally safe and do not cause hypoglycemia when used alone. -
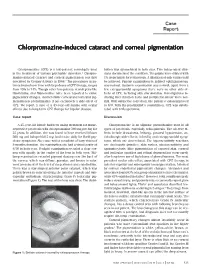
Chlorpromazine-Induced Cataract and Corneal Pigmentation
Case Report Chlorpromazine-induced cataract and corneal pigmentation Chlorpromazine (CPZ) is a low-potency neuroleptic used bution was symmetrical in both eyes. Two independent clini- in the treatment of various psychiatric disorders.1 Chlorpro- cians documented the condition. The pupils were dilated with mazine-induced cataract and corneal pigmentation was first 1% tropicamide for retinoscopy. A dilatation of only 4 mm could described by Greiner & Berry in 1964.2 The prevalence in pa- be achieved. Fundus examination by indirect ophthalmoscopy tients treated over time with large doses of CPZ therapy, ranges was normal. Systemic examination was normal; apart from a from 15% to 74%. Though other low-potency neuroleptics like few extrapyramidal symptoms there were no other side-ef- thioridazine and fluphenazine have been reported to cause fects of CPZ, including skin discoloration. Investigations in- pigmentary changes, characteristic corneal and lenticular pig- cluding liver function tests and peripheral smear were nor- mentation is predominantly if not exclusively a side-effect of mal. With subjective correction, the patient’s vision improved CPZ. We report a case of a 45-year-old female with ocular to 6/9. With the psychiatrist’s consultation, CPZ was substi- effects due to long-term CPZ therapy for bipolar disease. tuted with trifluoperazine. Case report Discussion A 45-year-old female had been taking treatment for manic- Chlorpromazine is an aliphatic phenothiazine used in all depressive psychosis with chlorpromazine 300 mg per day for types of psychosis, especially schizophrenia. The adverse ef- 25 years. In addition, she was found to have received lithium fects include drowsiness, lethargy, postural hypotension, an- 300 mg and haloperidol 5 mg, both twice daily for florid psy- ticholinergic side-effects, infertility and extrapyramidal symp- chotic symptoms. -

DESCRIPTION Tolazamide Is an Oral Blood-Glucose-Lowering Drug of the Sulfonylurea Class
TOLAZAMIDE- tolazamide tablet PD-Rx Pharmaceuticals, Inc. ---------- DESCRIPTION Tolazamide is an oral blood-glucose-lowering drug of the sulfonylurea class. Tolazamide is a white or creamy-white powder very slightly soluble in water and slightly soluble in alcohol. The chemical name is 1-(Hexahydro-1 H-azepin-1-yl)-3-( p-tolylsulfonyl)urea. Tolazamide has the following structural formula: Each tablet for oral administration contains 250 mg or 500 mg of tolazamide, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. CLINICAL PHARMACOLOGY Actions Tolazamide appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. The mechanism by which tolazamide lowers blood glucose during long-term administration has not been clearly established. With chronic administration in type II diabetic patients, the blood glucose-lowering effect persists despite a gradual decline in the insulin secretory response to the drug. Extrapancreatic effects may be involved in the mechanism of action of oral sulfonylurea hypoglycemic drugs. Some patients who are initially responsive to oral hypoglycemic drugs, including tolazamide tablets, may become unresponsive or poorly responsive over time. Alternatively, tolazamide tablets may be effective in some patients who have become unresponsive to one or more other sulfonylurea drugs. In addition to its blood glucose-lowering actions, tolazamide produces a mild diuresis by enhancement of renal free water clearance. Pharmacokinetics Tolazamide is rapidly and well absorbed from the gastrointestinal tract. Peak serum concentrations occur at 3 to 4 hours following a single oral dose of the drug. -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

Orange Book Patent Listing Dispute List
Patent Listing Disputes Current through September 10, 2021 Established Drug Product Due Date for NDA Holder NDA Holder NDA Number NDA Holder Strength(s) Relevant U.S. Patent Number(s) Type of Patent Claim Original Use Code (if applicable) Revised Use Code (if applicable) Dispute Outcome Name Response Response Date Disputes Not Related to epinephrine 205029 Belcher 1mg/mL 10,004,700 and 10,039,728 N/A N/A 7/24/2021 Pending Pending Use Code 7 mg 14 mg 8,168,209, 8,173,708, 8,283,379, Disputes Not Related to memantine hydrochloride 22525 Allergan Sales LLC N/A N/A 5/28/2021 5/28/2021 Patent Listing Updated 21 mg 8,329,752, 8,362,085 and 8,598,233 Use Code 28 mg 0.1 mg Disputes Not Related to epinephrine 201739 Kaleo Inc 0.15 mg 10,824,938 N/A N/A 2/28/2021 2/3/2021 Patent Listing Updated Use Code 0.3 mg Disputes Not Related to netarsudil and latanoprost 208259 Aerie Pharms Inc 0.02%/0.005% 10,654,844 N/A N/A 11/18/2020 10/30/2020 Patent Listing Updated Use Code Disputes Not Related to netarsudil 208254 Aerie Pharms Inc 0.02% 10,654,844 N/A N/A 11/18/2020 10/30/2020 Patent Listing Updated Use Code U-2869: IV Administration of cangrelor before U-2979: Method comprising IV administration PCI and continuous infusion for at least 2 of cangrelor before PCI then continuous hours or the duration of the PCI and, during infusion for at least 2 hours or the duration of cangrelor 204958 Chiesi 50 mg/vial 8,680,052 Method of Use 11/8/2020 11/3/2020 Patent Listing Updated or after the continuous infusion, PCI and, during or after continuous infusion, -

Association Between Serious Hypoglycemia and Calcium-Channel Blockers Used Concomitantly with Insulin Secretagogues
Research Letter | Diabetes and Endocrinology Association Between Serious Hypoglycemia and Calcium-Channel Blockers Used Concomitantly With Insulin Secretagogues Young Hee Nam, PhD; Colleen M. Brensinger, MS; Warren B. Bilker, PhD; James H. Flory, MD; Charles E. Leonard, MSCE, PharmD; Sean Hennessy, PhD, PharmD Introduction + Supplemental content Serious hypoglycemia is a major, potentially fatal adverse event caused by insulin secretagogues.1 Author affiliations and article information are Previous case reports suggested that calcium-channel blockers (CCBs) might reduce the risk of listed at the end of this article. serious hypoglycemia in patients with hyperinsulinemic hypoglycemia.2,3 However, the association of serious hypoglycemia and CCBs used with insulin secretagogues has remained unclear. Because insulin secretion by the pancreas is mediated by calcium influx in beta cells through calcium channels,4 we conducted a population-based observational study on the hypothesis that concomitant use of CCBs may be associated with reduced rates of serious hypoglycemia in insulin secretagogue users. Methods This self-controlled case series study was approved by the institutional review board of the University of Pennsylvania, which waived the requirement for informed consent because the use or disclosure of the protected health information involved no more than minimal risk to the privacy of individuals, and the research could not practicably be conducted without the waiver or alteration and without access to and use of the protected health information. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. We used claims data from the Medicaid programs of 5 US states (California, Florida, New York, Ohio, and Pennsylvania, encompassing more than a third of the nationwide Medicaid population), supplemented with Medicare claims for dual enrollees, from January 1, 1999, to December 31, 2011, and used the self- controlled case series design. -

Sulfonylureas
Therapeutic Class Overview Sulfonylureas INTRODUCTION In the United States (US), diabetes mellitus affects more than 30 million people and is the 7th leading cause of death (Centers for Disease Control and Prevention [CDC] 2018). Type 2 diabetes mellitus (T2DM) is the most common form of diabetes and is characterized by elevated fasting and postprandial glucose concentrations (American Diabetes Association [ADA] 2019[a]). It is a chronic illness that requires continuing medical care and ongoing patient self-management education and support to prevent acute complications and to reduce the risk of long-term complications (ADA 2019[b]). ○ Complications of T2DM include hypertension, heart disease, stroke, vision loss, nephropathy, and neuropathy (ADA 2019[a]). In addition to dietary and lifestyle management, T2DM can be treated with insulin, one or more oral medications, or a combination of both. Many patients with T2DM will require combination therapy (Garber et al 2019). Classes of oral medications for the management of blood glucose levels in patients with T2DM focus on increasing insulin secretion, increasing insulin responsiveness, or both, decreasing the rate of carbohydrate absorption, decreasing the rate of hepatic glucose production, decreasing the rate of glucagon secretion, and blocking glucose reabsorption by the kidney (Garber et al 2019). Pharmacologic options for T2DM include sulfonylureas (SFUs), biguanides, thiazolidinediones (TZDs), meglitinides, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogs, amylinomimetics, sodium-glucose cotransporter 2 (SGLT2) inhibitors, combination products, and insulin (Garber et al 2019). SFUs are the oldest of the oral antidiabetic medications, and all agents are available generically. The SFUs can be divided into 2 categories: first-generation and second-generation. -

Oregon Drug Use Review / Pharmacy & Therapeutics Committee
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Oregon Drug Use Review / Pharmacy & Therapeutics Committee Thursday, July 26, 2018 1:00 - 5:00 PM HP Conference Room 4070 27th Ct. SE Salem, OR 97302 MEETING AGENDA NOTE: Any agenda items discussed by the DUR/P&T Committee may result in changes to utilization control recommendations to the OHA. Timing, sequence and inclusion of agenda items presented to the Committee may change at the discretion of the OHA, P&T Committee and staff. The DUR/P&T Committee functions as the Rules Advisory Committee to the Oregon Health Plan for adoption into Oregon Administrative Rules 410-121-0030 & 410-121-0040 as required by 414.325(9). I. CALL TO ORDER 1:00 PM A. Roll Call & Introductions R. Citron (OSU) B. Conflict of Interest Declaration R. Citron (OSU) C. Approval of Agenda and Minutes T. Klein (Chair) D. Department Update T. Douglass (OHA) E. Legislative Update T. Douglass (OHA) F. Mental Health Clinical Advisory Group Discussion K. Shirley (MHCAG) 1:40 PM II. CONSENT AGENDA TOPICS T. Klein (Chair) A. P&T Methods B. CMS and State Annual Reports C. Quarterly Utilization Reports 1. Public Comment III. DUR ACTIVITIES 1:45 PM A. ProDUR Report R. Holsapple (DXC) B. RetroDUR Report D. Engen (OSU) C. Oregon State Drug Reviews K. Sentena (OSU) 1. A Review of Implications of FDA Expedited Approval Pathways, Including the Breakthrough Therapy Designation IV. -

Oral Health Fact Sheet for Dental Professionals Adults with Type 2 Diabetes
Oral Health Fact Sheet for Dental Professionals Adults with Type 2 Diabetes Type 2 Diabetes ranges from predominantly insulin resistant with relative insulin deficiency to predominantly an insulin secretory defect with insulin resistance, American Diabetes Association, 2010. (ICD 9 code 250.0) Prevalence • 23.6 million Americans have diabetes – 7.8% of U.S. population. Of these, 5.7 million do not know they have the disease. • 1.6 million people ≥20 years of age are diagnosed with diabetes annually. • 90–95% of diabetic patients have Type 2 Diabetes. Manifestations Clinical of untreated diabetes • High blood glucose level • Excessive thirst • Frequent urination • Weight loss • Fatigue Oral • Increased risk of dental caries due to salivary hypofunction • Accelerated tooth eruption with increasing age • Gingivitis with high risk of periodontal disease (poor control increases risk) • Salivary gland dysfunction leading to xerostomia • Impaired or delayed wound healing • Taste dysfunction • Oral candidiasis • Higher incidence of lichen planus Other Potential Disorders/Concerns • Ketoacidosis, kidney failure, gastroparesis, diabetic neuropathy and retinopathy • Poor circulation, increased occurrence of infections, and coronary heart disease Management Medication The list of medications below are intended to serve only as a guide to facilitate the dental professional’s understanding of medications that can be used for Type 2 Diabetes. Medical protocols can vary for individuals with Type 2 Diabetes from few to multiple medications. ACTION TYPE BRAND NAME/GENERIC SIDE EFFECTS Enhance insulin Sulfonylureas Glipizide (Glucotrol) Angioedema secretion Glyburide (DiaBeta, Fluconazoles may increase the Glynase, Micronase) hypoglycemic effect of glipizide Glimepiride (Amaryl) and glyburide. Tolazamide (Tolinase, Corticosteroids may produce Diabinese, Orinase) hyperglycemia. Floxin and other fluoroquinolones may increase the hypoglycemic effect of sulfonylureas. -

Download Product Insert (PDF)
PRODUCT INFORMATION Nateglinide Item No. 23320 CAS Registry No.: 105816-04-4 Formal Name: N-[[trans-4-(1-methylethyl)cyclohexyl] O OH carbonyl]-D-phenylalanine Synonyms: A-4166, SDZ-DJN 608 O MF: C H NO 19 27 3 N FW: 317.4 Purity: ≥98% H UV/Vis.: λmax: 207 nm Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Nateglinide is supplied as a crystalline solid. A stock solution may be made by dissolving the nateglinide in the solvent of choice. Nateglinide is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of nateglinide in these solvents is approximately 30 mg/ml. Description Nateglinide is a hypoglycemic agent.1-3 It induces insulin and somatostatin release from perfused rat pancreas when used at concentrations ranging from 0.03 to 3 μM.1 Nateglinide (3 µM) increases intracellular calcium levels in isolated rat pancreatic β cells, an effect that can be inhibited by the L-type calcium channel blocker nitrendipine (Item No. 17549). Nateglinide-induced secretion of insulin and somatostatin and calcium influx is also reversed by the potassium channel activator diazoxide (Item No. 14576). Oral administration of nateglinide (1.6 mg/kg) reduces blood glucose levels by 20% in fasted mice.2 It also decreases blood glucose levels in an oral glucose tolerance test in normal rats, genetically diabetic KK mice, and a rat model of diabetes induced by streptozotocin (STZ; Item No.