Altropine Sulfate, Statistical Review and Evaluation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
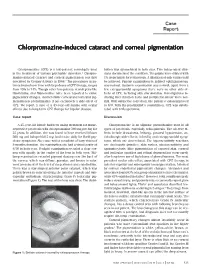
Chlorpromazine-Induced Cataract and Corneal Pigmentation
Case Report Chlorpromazine-induced cataract and corneal pigmentation Chlorpromazine (CPZ) is a low-potency neuroleptic used bution was symmetrical in both eyes. Two independent clini- in the treatment of various psychiatric disorders.1 Chlorpro- cians documented the condition. The pupils were dilated with mazine-induced cataract and corneal pigmentation was first 1% tropicamide for retinoscopy. A dilatation of only 4 mm could described by Greiner & Berry in 1964.2 The prevalence in pa- be achieved. Fundus examination by indirect ophthalmoscopy tients treated over time with large doses of CPZ therapy, ranges was normal. Systemic examination was normal; apart from a from 15% to 74%. Though other low-potency neuroleptics like few extrapyramidal symptoms there were no other side-ef- thioridazine and fluphenazine have been reported to cause fects of CPZ, including skin discoloration. Investigations in- pigmentary changes, characteristic corneal and lenticular pig- cluding liver function tests and peripheral smear were nor- mentation is predominantly if not exclusively a side-effect of mal. With subjective correction, the patient’s vision improved CPZ. We report a case of a 45-year-old female with ocular to 6/9. With the psychiatrist’s consultation, CPZ was substi- effects due to long-term CPZ therapy for bipolar disease. tuted with trifluoperazine. Case report Discussion A 45-year-old female had been taking treatment for manic- Chlorpromazine is an aliphatic phenothiazine used in all depressive psychosis with chlorpromazine 300 mg per day for types of psychosis, especially schizophrenia. The adverse ef- 25 years. In addition, she was found to have received lithium fects include drowsiness, lethargy, postural hypotension, an- 300 mg and haloperidol 5 mg, both twice daily for florid psy- ticholinergic side-effects, infertility and extrapyramidal symp- chotic symptoms. -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

Update on Surgical Management of Corneal Ulceration and Perforation
Romanian Journal of Ophthalmology, Volume 63, Issue 2, April-June 2019. pp:166-173 GENERAL ARTICLE Update on surgical management of corneal ulceration and perforation Stamate Alina-Cristina* **, Tătaru Călin Petru* ***, Zemba Mihail* **** *Department of Ophthalmology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania **Arena Med Clinic, Bucharest, Romania ***Clinical Hospital of Ophthalmologic Emergencies, Bucharest, Romania ****Department of Ophthalmology, “Dr. Carol Davila” Central Military Emergency University Hospital, Bucharest, Romania Correspondence to: Stamate Alina-Cristina, MD, Arena Med Clinic, Bucharest, 68 Basarabia Boulevard, Ap. 1, District 2, Bucharest, Romania, Mobile phone: +40737 027 067, E-mail: [email protected] Accepted: May 28th, 2019 Abstract Corneal ulcerations are a medical emergency, and in recalcitrant cases, leading to perforation, a surgical ophthalmological emergency. The urgency of the treatment is dictated by the necessity of preventing complications that can lead to serious ocular morbidities. Medical treatment represents the first therapeutic approach and is a defining step in the further management of a patient with corneal ulceration. Multiple surgical strategies are available, but the option depends on the etiology and parameters of the ulceration: size, depth, and location. Keywords: corneal ulceration, corneal perforation, tissue adhesives, cross-linking, amniotic membrane, conjunctival flap, keratoplasty Introduction reepithelialization by using preservative-free lubricants, -

Eleventh Edition
SUPPLEMENT TO April 15, 2009 A JOBSON PUBLICATION www.revoptom.com Eleventh Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO Supported by an unrestricted grant from Alcon, Inc. 001_ro0409_handbook 4/2/09 9:42 AM Page 4 TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Viitreous & Retiina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Blow-Out Fracture................................................ 6 Asteroid Hyalosis ................................................33 Acquired Ptosis ................................................... 7 Retinal Arterial Macroaneurysm............................34 Acquired Entropion ............................................. 9 Retinal Emboli.....................................................36 Verruca & Papilloma............................................11 Hypertensive Retinopathy.....................................37 Idiopathic Juxtafoveal Retinal Telangiectasia...........39 CONJUNCTIVA & SCLERA Ocular Ischemic Syndrome...................................40 Scleral Melt ........................................................13 Retinal Artery Occlusion ......................................42 Giant Papillary Conjunctivitis................................14 Conjunctival Lymphoma .......................................15 NEURO-OPHTHALMIC DISEASE Blue Sclera .........................................................17 Dorsal Midbrain Syndrome ..................................45 -

This May Be the Author's Version of a Work That Was Submitted/Accepted
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Sander, Beata, Collins, Michael,& Read, Scott (2014) The effect of topical adrenergic and anticholinergic agents on the choroidal thickness of young healthy adults. Experimental Eye Research, 128, pp. 181-189. This file was downloaded from: https://eprints.qut.edu.au/78994/ c Consult author(s) regarding copyright matters This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] License: Creative Commons: Attribution-Noncommercial-No Derivative Works 4.0 Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.exer.2014.10.003 Experimental Eye Research 128 (2014) 181e189 Contents lists available at ScienceDirect Experimental Eye Research journal homepage: www.elsevier.com/locate/yexer The effect of topical adrenergic and anticholinergic agents on the choroidal thickness of young healthy adults * Beata P. -

Management of Corneal Perforation Vishal Jhanji, MD,1,2,3 Alvin L
SURVEY OF OPHTHALMOLOGY VOLUME 56 NUMBER 6 NOVEMBER–DECEMBER 2011 MAJOR REVIEW Management of Corneal Perforation Vishal Jhanji, MD,1,2,3 Alvin L. Young, MMedSc (Hons), FRCSI,3 Jod S. Mehta, MD,4 Namrata Sharma, MD,5 Tushar Agarwal, MD,5 and Rasik B. Vajpayee, MS, FRCS (Edin), FRANZCO1,5,6 1Centre for Eye Research Australia, University of Melbourne, Australia; 2Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong; 3Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong; 4Singapore National Eye Centre, Singapore; 5Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India; and 6Royal Victorian Eye and Ear Hospital, Melbourne, Australia Abstract. Corneal perforation may be associated with prolapse of ocular tissue and requires prompt diagnosis and treatment. Although infectious keratitis is an important cause, corneal xerosis and collagen vascular diseases should be considered in the differential diagnosis, especially in cases that do not respond to conventional medical therapy. Although medical therapy is a useful adjunct, a surgical approach is required for most corneal perforations. Depending on the size and location of the corneal perforation, treatment options include gluing, amniotic membrane transplantation, and corneal transplantation. (Surv Ophthalmol 56:522--538, 2011. Ó 2011 Elsevier Inc. All rights reserved.) Key words. corneal perforation diagnosis keratoplasty management patch graft therapeutic keratoplasty I. Introduction The selection of an appropriate treatment option is Corneal perforation is a cause of ocular morbidity mostly guided by size and location of the perfora- and profound visual loss.13,119 It is the end result of tion and the status of the underlying disease. -
Guidelines for Universal Eye Screening in Newborns Including RETINOPATHY of Prematurity
GUIDELINES FOR UNIVERSAL EYE SCREENING IN NEWBORNS INCLUDING RETINOPATHY OF PREMATURITY RASHTRIYA BAL SWASthYA KARYAKRAM Ministry of Health & Family Welfare Government of India June 2017 MESSAGE The Ministry of Health & Family Welfare, Government of India, under the National Health Mission launched the Rashtriya Bal Swasthya Karyakram (RBSK), an innovative and ambitious initiative, which envisages Child Health Screening and Early Intervention Services. The main focus of the RBSK program is to improve the quality of life of our children from the time of birth till 18 years through timely screening and early management of 4 ‘D’s namely Defects at birth, Development delays including disability, childhood Deficiencies and Diseases. To provide a healthy start to our newborns, RBSK screening begins at birth at delivery points through comprehensive screening of all newborns for various defects including eye and vision related problems. Some of these problems are present at birth like congenital cataract and some may present later like Retinopathy of prematurity which is found especially in preterm children and if missed, can lead to complete blindness. Early Newborn Eye examination is an integral part of RBSK comprehensive screening which would prevent childhood blindness and reduce visual and scholastic disabilities among children. Universal newborn eye screening at delivery points and at SNCUs provides a unique opportunity to identify and manage significant eye diseases in babies who would otherwise appear healthy to their parents. I wish that State and UTs would benefit from the ‘Guidelines for Universal Eye Screening in Newborns including Retinopathy of Prematurity’ and in supporting our future generation by providing them with disease free eyes and good quality vision to help them in their overall growth including scholastic achievement. -

Acepromazine and Chlorpromazine As Pharmaceutical-Grade Alternatives to Chlorprothixene for Pupillary Light Reflex Imaging in Mice
Journal of the American Association for Laboratory Animal Science Vol 59, No 2 Copyright 2020 March 2020 by the American Association for Laboratory Animal Science Pages 197–203 Acepromazine and Chlorpromazine as Pharmaceutical-grade Alternatives to Chlorprothixene for Pupillary Light Reflex Imaging in Mice Samantha S Eckley,1 Jason S Villano,1 Nora S Kuo,1 and Kwoon Y Wong2,* Studies of visual responses in isoflurane-anesthetized mice often use the sedative chlorprothixene to decrease the amount of isoflurane used because excessive isoflurane could adversely affect light-evoked responses. However, data are not available to justify the use of this nonpharmaceutical-grade chemical. The current study tested whether pharmaceutical-grade sedatives would be appropriate alternatives for imaging pupillary light reflexes. Male 15-wk-old mice were injected intraperitoneally with 1 mg/kg chlorprothixene, 5 mg/kg acepromazine, 10 mg/kg chlorpromazine, or saline. After anesthetic induction, anes- thesia maintenance used 0.5% and 1% isoflurane for sedative- and saline-injected mice, respectively. A photostimulus (16.0 log photons cm−2 s−1; 470 nm) was presented to the right eye for 20 min, during which the left eye was imaged for consensual pupillary constriction and involuntary pupil drift. Time to immobilization, loss of righting reflex, physiologic parameters, gain of righting reflex, and degree of recovery were assessed also. The sedative groups were statistically indistinguishable for all measures. By contrast, pupillary drift occurred far more often in saline-treated mice than in the sedative groups. Fur- thermore, saline-treated mice took longer to reach maximal pupil constriction than all sedative groups and had lower heart rates compared with chlorpromazine- and chlorprothixene-sedated mice. -

Tropicamide Tropicamide Eye Drops
Minims® Tropicamide Tropicamide Eye Drops Consumer Medicine Information Tell your doctor if you have or What is in this leaflet Before you use Minims have had any of the following Tropicamide This leaflet answers some common medical conditions: questions about Minims * glaucoma (high pressure in the Tropicamide, including how to use When you must not use it eye) the eye drops. * fast heartbeat or any heart Do not use Minims Tropicamide if It does not contain all the available condition or had heart surgery. you have an allergy to: information. It does not take the Tell your doctor if you are place of talking to your doctor or * any medicine containing pregnant or are breast-feeding. pharmacist. tropicamide Your doctor will discuss with you * any of the ingredients listed at the All medicines have benefits and the risks and benefits involved. risks. Your doctor has weighed the end of this leaflet. Tell your doctor if you wear risks of you using Minims Some of the symptoms of an allergic contact lenses. Tropicamide against the benefits they reaction may include: You should not wear contact lenses expect it will have for you. * shortness of breath while using this medicine. If you have any concerns about * wheezing or difficulty breathing using this medicine, ask your * swelling of the face, lips, tongue If you have not told your doctor doctor or pharmacist. about any of the above, tell or other parts of the body him/her before you start using Keep this leaflet with the medicine. * rash, itching or hives on the skin. -

Practical Tips for Managing Myopia
MYOPIA MANAGEMENT Practical tips for managing myopia Michael Morton This article presents a summary of Online Education Coordinator: practical approaches to diagnosing Brien Holden Vision myopia, myopia management Institute, Sydney, Australia. (with particular attention to low resource settings), reviewing myopia progression, and collecting data for myopia management programmes. Ling Lee Research Officer/ Optometrist: Part 1 Diagnosing and prescribing Brien Holden Vision Institute, Sydney, for myopia Australia. While myopia might be initially detected by a patient EDGARDO CONTRERAS, COURTESY OF IAPB (e.g. reporting distance blur), or an adult observing Refraction is the first step. MEXICO behaviour changes in a child (e.g. squinting or • Monocular estimate method (MEM) retinoscopy. viewing things closer than expected), myopia is generally An objective method to determine a child’s diagnosed by an eye care professional. accommodative (near focussing) status at near. Priya Morjaria Equipment Retinoscopy should be conducted with a near target. Research Fellow: Accommodative facility. A subjective method to Department of The minimum required equipment to diagnose myopia • Clinical Research, and assess progression includes: assess accommodation function (ability of eye to London School focus at near). A high-contrast distance visual acuity (VA) chart (e.g., of Hygiene and • • Subjective phorias. A subjective method to Tropical Medicine, Snellen, logMAR, E, or LEA) determine whether the eyes prefer to converge in or International Centre • A room or space where the viewing distance for VA diverge out, at distance and near. for Eye Health, is at least 3m/10ft. The chart should be well lit and • Vergence reserves. A subjective method that London, UK. calibrated for the working distance measures the eyes’ ability to converge in and • Occluder (ideally with pinhole occluder) diverge out. -

Treatment of Peripheral Corneal Ulcers by Limbal Conjunctivectomy
Brit. 7. Ophthal. (I 976) 6o, 713 Br J Ophthalmol: first published as 10.1136/bjo.60.10.713 on 1 October 1976. Downloaded from Treatment of peripheral corneal ulcers by limbal conjunctivectomy FRED M. WILSON II, MERRILL GRAYSON, AND FORREST D. ELLIS From the Department of Ophthalmology, Indiana University School of Medicine, Indianapolis, Indiana Peripheral corneal ulcers can still pose difficult appear within the ulcer about one week later, followed clinical problems despite therapeutic advances such in a few days by superficial vascularization. The ulcer as specific antimicrobial agents, collagenase inhibi- had healed and epithelialized three weeks after surgery tors, heparin, ocular lubricants, biological adhe- (Fig. ib) and L-cysteine and heparin drops were con- sives, and soft contact lenses. This paper reports tinued for three weeks after it had healed. The ulcer has the healing of several types of progressive marginal ulcers after the excision and recession of adjacent limbal conjunctiva (limbal conjunctivectomy), in some cases after other modes of treatment had been unsuccessful. copyright. Case reports CASE I, MOOREN )S ULCER A 54-year-old Black woman developed a severely painful, largely non-infiltrative ulcer of the nasal left cornea. The ulcer had an overhanging central edge and progressed circumferentially during a period of one year to involve nearly the entire comeal periphery http://bjo.bmj.com/ (Fig. ia). Progression occurred despite treatment at various times with acetylcysteine drops, L-cysteine drops, topical and subconjunctival heparin, artificial soft contact a short trial of tears, lens, topical cortico- (Ia.) steroids, and a trial without any treatment to minimize the possibility of overtreatment. -

208151Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 208151Orig1s000 SUMMARY REVIEW NDA 208151, ISOPTO Atropine (atropine sulfate ophthalmic solution) 1% Cycloplegia, Mydriasis, Penalization of the healthy eye in the treatment of amblyopia Division Director Summary Review Material Reviewed/Consulted OND Action Package, including: Names of discipline reviewers Medical Officer Review Wiley Chambers, William Boyd 9/13/2016 CDTL Review William Boyd, Wiley Chambers 11/29/2016 Statistical Review Abel Eshete, Yan Wang 11/8/2016 Pharmacology Toxicology Review Aaron Ruhland, Lori Kotch 11/7/2016 CMC Review Haripada Sanker, Milton Sloan, Maotang Zhou, Denise Miller, Vidya Pai, Om Anand, Erin Andrews, Chunchun Zhang, Paul Perdue 10/12/2016, 11/10/2016 Clinical Pharmacology Review Abhay Joshi, Philip Colangelo 11/9/2016 OPDP/DPDP Carrie Newcomer 11/7/2016 OSI/DGCPC N/A Proprietary Name Michelle Rutledge, Yelena Maslov, Lubna Merchant 6/1/2016 Conditionally acceptable letter Todd Bridges 6/2/2016 OSE/DMEPA Michelle Rutledge, Yelena Maslov 6/8/2016 OSE/DDRE N/A OSE/DRISK N/A Project Manager Michael Puglisi OND=Office of New Drugs CDTL=Cross-Discipline Team Leader OSI/DGCPC=Office of Scientific Investigations/Division of Good Clinical Practice Compliance OPDP/DPDP=Office of Prescription Drug Promotion/Division of Prescription Drug Promotion OSE= Office of Surveillance and Epidemiology DMEPA=Division of Medication Error Prevention and Analysis DDRE= Division of Drug Risk Evaluation DRISK=Division of Risk Management Page 2 of 24 Reference ID: 4021108 NDA 208151, ISOPTO Atropine (atropine sulfate ophthalmic solution) 1% Cycloplegia, Mydriasis, Penalization of the healthy eye in the treatment of amblyopia Division Director Summary Review Signatory Authority Review Template 1.