90-Day Extended Supply Medications (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME INCRUSE ELLIPTA Umeclidinium (as bromide), 62.5 micrograms, powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 55 micrograms umeclidinium (equivalent to 65 micrograms of umeclidinium bromide). This corresponds to a pre-dispensed dose of 62.5 micrograms of umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide). Excipient with known effect: Each delivered dose contains approximately 12.5 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for Inhalation White powder in a grey inhaler (Ellipta) with a light green mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Incruse Ellipta is indicated as a long-term once daily maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). 4.2 Dose and method of administration Dose Adults Incruse Ellipta (umeclidinium 62.5 micrograms) should be taken as one inhalation once daily by the orally inhaled route. Incruse Ellipta should be taken at the same time every day. Do not use Incruse Ellipta more than once every 24 hours. Special populations Elderly population No dosage adjustment is required in patients over 65 years (see 5.2 Pharmacokinetics properties– Special patient populations). 1 Renal impairment No dosage adjustment is required in patients with renal impairment (see 5.2 Pharmacokinetics properties – Special patient populations). Hepatic impairment No dosage adjustment is required in patients with mild or moderate hepatic impairment. Incruse Ellipta has not been studied in patients with severe hepatic impairment (see 5.2 Pharmacokinetics properties– Special patient populations). -
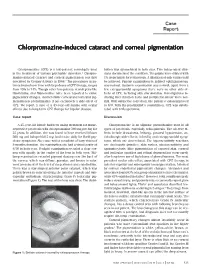
Chlorpromazine-Induced Cataract and Corneal Pigmentation
Case Report Chlorpromazine-induced cataract and corneal pigmentation Chlorpromazine (CPZ) is a low-potency neuroleptic used bution was symmetrical in both eyes. Two independent clini- in the treatment of various psychiatric disorders.1 Chlorpro- cians documented the condition. The pupils were dilated with mazine-induced cataract and corneal pigmentation was first 1% tropicamide for retinoscopy. A dilatation of only 4 mm could described by Greiner & Berry in 1964.2 The prevalence in pa- be achieved. Fundus examination by indirect ophthalmoscopy tients treated over time with large doses of CPZ therapy, ranges was normal. Systemic examination was normal; apart from a from 15% to 74%. Though other low-potency neuroleptics like few extrapyramidal symptoms there were no other side-ef- thioridazine and fluphenazine have been reported to cause fects of CPZ, including skin discoloration. Investigations in- pigmentary changes, characteristic corneal and lenticular pig- cluding liver function tests and peripheral smear were nor- mentation is predominantly if not exclusively a side-effect of mal. With subjective correction, the patient’s vision improved CPZ. We report a case of a 45-year-old female with ocular to 6/9. With the psychiatrist’s consultation, CPZ was substi- effects due to long-term CPZ therapy for bipolar disease. tuted with trifluoperazine. Case report Discussion A 45-year-old female had been taking treatment for manic- Chlorpromazine is an aliphatic phenothiazine used in all depressive psychosis with chlorpromazine 300 mg per day for types of psychosis, especially schizophrenia. The adverse ef- 25 years. In addition, she was found to have received lithium fects include drowsiness, lethargy, postural hypotension, an- 300 mg and haloperidol 5 mg, both twice daily for florid psy- ticholinergic side-effects, infertility and extrapyramidal symp- chotic symptoms. -

Long Term Follow up of Mirabegron - a Real Life- Pragmatic Experience
Central Journal of Urology and Research Bringing Excellence in Open Access Research Article *Corresponding author Haitham Abdelmoteleb, Bristol Urological Institute, Southmead Hospital, Bristol, BS10 5NB, UK, Tel: 440-7884- Long Term Follow Up of 827995; Email: Submitted: 04 June 2016 Mirabegron - A Real Life- Accepted: 22 August 2016 Published: 24 August 2016 ISSN: 2379-951X Pragmatic Experience Copyright Haitham Abdelmoteleb*, Musaab Yassin, and Hashim Hashim © 2016 Abdelmoteleb et al. Bristol Urological Institute, South mead Hospital, UK OPEN ACCESS Abstract Keywords • Mirabegron Objectives: To evaluate the efficacy and tolerability of Mirabegron for the • Beta-3 agonist treatment of overactive bladder symptoms in a real life, long term follow up study • Overactive bladder syndrome conducted in a tertiary referral centre. • Long term Methods: A structured telephone questionnaire of patients was conducted in order to evaluate the efficacy and tolerability of Mirabegron. Patients who were initially prescribed and responded to Mirabegron 50mg once daily between 6/2013 and 9/2013, were interviewed to see if they were compliant with treatment, continue to respond to treatment and if they discontinued treatment. Results: Follow-up was for a mean of 11.7 months. At short-term follow-up, 20/39 patients responded to treatment. In the long term follow-up, 18/20 patients were still using Mirabegron. 2/20 patients discontinued because of lack of efficacy. Overall, the main reasons for discontinuation of Mirabegron after trying it for a mean of 5.3 months, was lack of efficacy and adverse events. The majority of AEs were mild in severity and few were serious. Other reasons include the lack of further prescription from general practitioners. -

Betmiga, INN-Mirabegron
EMA/591015/2015 EMEA/H/C/002388 EPAR summary for the public Betmiga mirabegron This is a summary of the European public assessment report (EPAR) for Betmiga. It explains how the Committee for Medicinal Products for Human Use (CHMP) assessed the medicine to reach its opinion in favour of granting a marketing authorisation and its recommendations on the conditions of use for Betmiga. What is Betmiga? Betmiga is a medicine containing the active substance mirabegron. It is available as prolonged-release tablets (25 mg, 50 mg). ‘Prolonged-release’ means that mirabegron is released slowly from the tablet over a few hours. What is Betmiga used for? Betmiga is used in adults with overactive bladder syndrome. It is used to treat certain symptoms of the condition: urgency (sudden urge to urinate), increased urinary frequency (the need to urinate frequently) and urge incontinence (involuntary leakage of urine from the bladder when a sudden strong need to urinate is felt). The medicine can only be obtained with a prescription. How is Betmiga used? The recommended dose of Betmiga is 50 mg once a day. In patients who have reduced kidney or liver function the doctor may need to prescribe a lower dose or avoid the use of Betmiga, especially in patients taking certain other medicines. For full details, see the package leaflet (also part of the EPAR). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2015. -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

T 1635/09 T 1635/09 T 1635/09 (Verfahrenssprache) (Translation) (Traduction)
542 Amtsblatt EPA Official Journal EPO Journal officiel OEB 11/2011 Entscheidung der Technischen Decision of Technical Board Décision de la Chambre de Beschwerdekammer 3.3.02 of Appeal 3.3.02 dated recours technique 3.3.02 en date vom 27. Oktober 2010 27 October 2010 du 27 octobre 2010 T 1635/09 T 1635/09 T 1635/09 (Verfahrenssprache) (Translation) (Traduction) Zusammensetzung der Kammer: Composition of the Board: Composition de la Chambre : Vorsitzender: Chairman: Président : U. Oswald U. Oswald U. Oswald Mitglieder: Members: Membres : A. Lindner, L. Bühler A. Lindner, L. Bühler A. Lindner, L. Bühler Patentinhaber/Beschwerdeführer: Patent proprietor/Appellant: Titulaire/requérant : Bayer Schering Pharma Bayer Schering Pharma Bayer Schering Pharma Aktiengesellschaft Aktiengesellschaft Aktiengesellschaft Einsprechender 01/ Opponent 01/Appellant: Opposant 01/requérant : Beschwerdeführer: STRAGEN PHARMA SA STRAGEN PHARMA SA STRAGEN PHARMA SA Einsprechender 02/ Opponent 02/Appellant: Opposant 02/requérant : Beschwerdeführer: Laboratorios Léon Farma, S.A. Laboratorios Léon Farma, S.A. Laboratorios Léon Farma, S.A. Einsprechender 03/ Opponent 03/Appellant: Opposant 03/requérant : Beschwerdeführer: Sandoz AG Sandoz AG Sandoz AG Einsprechender 04/ Opponent 04/Party to the Opposant 04/partie à la procédure : Verfahrensbeteiligter: proceedings: Helm AG Helm AG Helm AG Stichwort: Headword: Référence : Zusammensetzung für Empfängnisver- Composition for contraception/BAYER Composition contraceptive/BAYER hütung/BAYER SCHERING PHARMA AG SCHERING PHARMA -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Frailty & Cognihon: Management of OAB in Elderly & Frail Pahents
Frailty & Cogni.on: Management of OAB in Elderly & Frail Pa.ents MR. RAHUL MISTRY CONSULTANT UROLOGICAL SURGEON POGP CONFERENCE NOVEMBER 2016 Topics • What are LUTS? • Symptom definiDons • The impact of OAB on paents • Management of OAB • The challenge of treang OAB • The Ancholinergic burden • Novel treatment of OAB Change of Terminology • LUTS = Lower urinary tract symptoms • LUTS instead of “prostasm” • Storage instead of “irritave” • Voiding instead of “obstrucDve” What are LUTS Post-micturion Storage symptoms Voiding symptoms symptoms DayDme frequency Slow stream Post-micturiDon Nocturia Spraying dribbling Incomplete Urgency IntermiUency emptying Urinary Hesitancy inconnence Straining Terminal dribbling Definions • Urgency - sudden compelling desire to pass urine which is difficult to defer • Urinary inconnence • any involuntary leakage of urine (urge / stress) • may need to be disDnguished from sweang or vaginal discharge • Increased dayme frequency - the complaint by the paent who considers that he/she voids too o]en by day • Nocturia - individual has to wake at night one or more Dmes to void • ICS DefiniDons, Abrams P et al. Neurourol Urodyn; 21:167-178 (2002) Defini.on of Overac.ve Bladder (OAB) (Internaonal Connence Society) 1. Abrams P et al. Neurourol Urodyn 2002;21:167-178 Mrs W. E. Terrible, your classic pa.ent? Age: 64 year old woman Occupaon: Recently reDred from an office job Symptoms: • Urinary frequency (14−15 Dmes a day) • Urge inconDnence (daily) • Nocturia (3 Dmes a night) THe impact of OAB on pa.ents How do OAB symptoms affect pa.ents? haven’t slept depressing like a baby negave lose control locked in embarrassing exhausted no control terrible so red I keep it xxx secret Quotes from a video of real-life OAB paents talking about how OAB has affected their lives. -

UFC PROHIBITED LIST Effective June 1, 2021 the UFC PROHIBITED LIST
UFC PROHIBITED LIST Effective June 1, 2021 THE UFC PROHIBITED LIST UFC PROHIBITED LIST Effective June 1, 2021 PART 1. Except as provided otherwise in PART 2 below, the UFC Prohibited List shall incorporate the most current Prohibited List published by WADA, as well as any WADA Technical Documents establishing decision limits or reporting levels, and, unless otherwise modified by the UFC Prohibited List or the UFC Anti-Doping Policy, Prohibited Substances, Prohibited Methods, Specified or Non-Specified Substances and Specified or Non-Specified Methods shall be as identified as such on the WADA Prohibited List or WADA Technical Documents. PART 2. Notwithstanding the WADA Prohibited List and any otherwise applicable WADA Technical Documents, the following modifications shall be in full force and effect: 1. Decision Concentration Levels. Adverse Analytical Findings reported at a concentration below the following Decision Concentration Levels shall be managed by USADA as Atypical Findings. • Cannabinoids: natural or synthetic delta-9-tetrahydrocannabinol (THC) or Cannabimimetics (e.g., “Spice,” JWH-018, JWH-073, HU-210): any level • Clomiphene: 0.1 ng/mL1 • Dehydrochloromethyltestosterone (DHCMT) long-term metabolite (M3): 0.1 ng/mL • Selective Androgen Receptor Modulators (SARMs): 0.1 ng/mL2 • GW-1516 (GW-501516) metabolites: 0.1 ng/mL • Epitrenbolone (Trenbolone metabolite): 0.2 ng/mL 2. SARMs/GW-1516: Adverse Analytical Findings reported at a concentration at or above the applicable Decision Concentration Level but under 1 ng/mL shall be managed by USADA as Specified Substances. 3. Higenamine: Higenamine shall be a Prohibited Substance under the UFC Anti-Doping Policy only In-Competition (and not Out-of- Competition). -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: PRODUCT IDENTIFICATION PRODUCT NAME DHEA (Prasterone) (Micronized) PRODUCT CODE 0733 SUPPLIER MEDISCA Inc. Tel.: 1.800.932.1039 | Fax.: 1.855.850.5855 661 Route 3, Unit C, Plattsburgh, NY, 12901 3955 W. Mesa Vista Ave., Unit A-10, Las Vegas, NV, 89118 6641 N. Belt Line Road, Suite 130, Irving, TX, 75063 MEDISCA Pharmaceutique Inc. Tel.: 1.800.665.6334 | Fax.: 514.338.1693 4509 Rue Dobrin, St. Laurent, QC, H4R 2L8 21300 Gordon Way, Unit 153/158, Richmond, BC V6W 1M2 MEDISCA Australia PTY LTD Tel.: 1.300.786.392 | Fax.: 61.2.9700.9047 Unit 7, Heritage Business Park 5-9 Ricketty Street, Mascot, NSW 2020 EMERGENCY PHONE CHEMTREC Day or Night Within USA and Canada: 1-800-424-9300 NSW Poisons Information Centre: 131 126 USES Adjuvant; Androgen SECTION 2: HAZARDS IDENTIFICATION GHS CLASSIFICATION Toxic to Reproduction (Category 2) PICTOGRAM SIGNAL WORD Warning HAZARD STATEMENT(S) Reproductive effector, prohormone. Suspected of damaging fertility or the unborn child. May cause harm to breast-fed children. Causes serious eye irritation. Causes skin and respiratory irritation. Very toxic to aquatic life with long lasting effects. AUSTRALIA-ONLY HAZARDS Not Applicable. PRECAUTIONARY STATEMENT(S) Prevention Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Do not breathe dusts or mists. Do not eat, drink or smoke when using this product. Avoid contact during pregnancy/while nursing. Wear protective gloves, protective clothing, eye protection, face protection. Avoid release to the environment. Response IF ON SKIN (HAIR): Wash with plenty of water. -
Study Protocol
CLINICAL STUDY PROTOCOL Study Title: An International Phase 3, Randomized, Double-Blind, Placebo- and Active (Tolterodine)-Controlled Multicenter Study to Evaluate the Safety and Efficacy of Vibegron in Patients with Symptoms of Overactive Bladder Protocol Number: RVT-901-3003 Compound Name and/or Vibegron Number: Indication Treatment of Overactive Bladder Sponsor: Urovant Sciences GmbH Viaduktstrasse 8 4051 Basel Switzerland Development Phase: 3 Regulatory Identifier(s): IND# 106,410 EudraCT# 2017-003293-14 Current Version and Version 3.0; 15 NOV 2018 Effective Date: Previous Version(s) and Version 2.1; 12 FEB 2018 Effective Date(s): Version 2.0; 30 JAN 2018 Version 1.2; 01 NOV 2017 Version 1.1; 05 OCT 2017 Version 1.0; 29 SEP 2017 Study Director: , , Clinical Development Confidentiality Statement The information contained in this document, particularly unpublished data, is the property or under control of Urovant Sciences GmbH, and is provided to you in confidence as an investigator, potential investigator or consultant, for review by you, your staff, and an applicable Institutional Review Board or Independent Ethics Committee. The information is only to be used by you in connection with authorized clinical studies of the investigational drug described in the protocol. You will not disclose any of the information to others without written authorization from Urovant Sciences GmbH. except to the extent necessary to obtain informed consent from those persons to whom the drug may be administered. Confidential Page | 1 NCT Number: NCT03492281 This NCT number has been applied to the document for purposes of posting on clinicaltrials.gov Clinical Study Protocol RVT-901-3003 Urovant Sciences GmbH Effective: 15 NOV 2018 SUMMARY OF CHANGES Version Location Description of Change 3.0 Global Minor typographical/formatting errors were corrected. -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020