New Opportunities for Conservation of Handfishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation of Handfish and Their Habitats – Annual Report Tim Lynch, Tyson Bessell, Alexander Hormann, Carlie Devine and Neville Barrett
Conservation of handfish and their habitats – annual report Tim Lynch, Tyson Bessell, Alexander Hormann, Carlie Devine and Neville Barrett Project A10 – Conservation of spotted handfish 28 February 2019 Milestone 4– Research Plan 4 (2018) www.nespmarine.edu.au Enquiries should be addressed to: Dr Tim P. Lynch Senior Research Scientist CSIRO Castray Esplanade [email protected] Project Leader’s Distribution List Derwent Estuary Program Ursula Taylor Zoo and Aquarium Association (ZAA) Craig Thorburn Natural Resource Management (NRM) Nepelle Crane South MAST Ian Ross Royal Yacht Club of Tasmania Nick Hutton Derwent Sailing Squadron Shaun Tiedemann The Handfish Recovery Team (HRT) See list below Marine and Freshwater Species Conservation Section Wildlife, Heritage and Marine Division Department of the Environment and Energy (DoEE) Threatened Species Policy and Andrew Crane Conservation Advice Branch Department of Primary Industries, Parks, Water and Environment (DPIPWE) Office of the Threatened Species Commissioner (DoEE) The project will also report its findings on a semi-annual basis to the National Handfish Recovery Team (NHRT) – see below. This is a governance body that is constituted between the Tasmanian State and the Commonwealth government with other interested parties: Department of the Environment and Energy (Commonwealth) Department of Primary Industries, Parks, Water and Andrew Crane Environment (Tas) CSIRO scientist, running current surveys and substrate trials Tim Lynch (Chair) University of Tasmania, handfish research Neville -

Rare Birds of California Now Available! Price $54.00 for WFO Members, $59.99 for Nonmembers
Volume 40, Number 3, 2009 The 33rd Report of the California Bird Records Committee: 2007 Records Daniel S. Singer and Scott B. Terrill .........................158 Distribution, Abundance, and Survival of Nesting American Dippers Near Juneau, Alaska Mary F. Willson, Grey W. Pendleton, and Katherine M. Hocker ........................................................191 Changes in the Winter Distribution of the Rough-legged Hawk in North America Edward R. Pandolfino and Kimberly Suedkamp Wells .....................................................210 Nesting Success of California Least Terns at the Guerrero Negro Saltworks, Baja California Sur, Mexico, 2005 Antonio Gutiérrez-Aguilar, Roberto Carmona, and Andrea Cuellar ..................................... 225 NOTES Sandwich Terns on Isla Rasa, Gulf of California, Mexico Enriqueta Velarde and Marisol Tordesillas ...............................230 Curve-billed Thrasher Reproductive Success after a Wet Winter in the Sonoran Desert of Arizona Carroll D. Littlefield ............234 First North American Records of the Rufous-tailed Robin (Luscinia sibilans) Lucas H. DeCicco, Steven C. Heinl, and David W. Sonneborn ........................................................237 Book Reviews Rich Hoyer and Alan Contreras ...........................242 Featured Photo: Juvenal Plumage of the Aztec Thrush Kurt A. Radamaker .................................................................247 Front cover photo by © Bob Lewis of Berkeley, California: Dusky Warbler (Phylloscopus fuscatus), Richmond, Contra Costa County, California, 9 October 2008, discovered by Emilie Strauss. Known in North America including Alaska from over 30 records, the Dusky is the Old World Warbler most frequent in western North America south of Alaska, with 13 records from California and 2 from Baja California. Back cover “Featured Photos” by © Kurt A. Radamaker of Fountain Hills, Arizona: Aztec Thrush (Ridgwayia pinicola), re- cently fledged juvenile, Mesa del Campanero, about 20 km west of Yecora, Sonora, Mexico, 1 September 2007. -
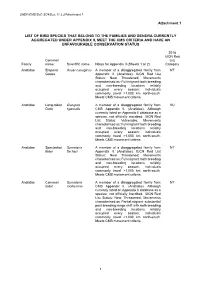
Attachment 1 LIST of BIRD SPECIES THAT BELONG to the FAMILIES
UNEP/CMS/ScC-SC4/Doc.11.3.2/Attachment 1 Attachment 1 LIST OF BIRD SPECIES THAT BELONG TO THE FAMILIES AND GENERA CURRENTLY AGGREGATED UNDER APPENDIX II, MEET THE CMS CRITERIA AND HAVE AN UNFAVOURABLE CONSERVATION STATUS 2018 IUCN Red Common List Family name Scientific name Notes for Appendix II (Sheets 1 or 2) Category Anatidae Emperor Anser canagicus A member of a disaggregated family from NT Goose Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Long-tailed Clangula A member of a disaggregated family from VU Duck hyemalis CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Vulnerable; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Spectacled Somateria A member of a disaggregated family from NT Eider fischeri Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Common Somateria A member of a disaggregated family from NT Eider mollissima CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Near Threatened; Movements characterised as: Partial migrant: substantial post-breeding range shift with both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Proposed Development Information to Accompany
ENVIRONMENTAL IMPACT STATEMENT TO ACCOMPANY DRAFT AMENDMENT NO.6 TO D’ENTRECASTEAUX CHANNEL MARINE FARMING DEVELOPMENT PLAN FEBRUARY 2002 PROPONENT: TASSAL OPERATIONS PTY LTD Glossary ADCP Acoustic Doppler Current Profiler AGD Amoebic Gill Disease ASC Aquaculture Stewardship Council BAP Best Aquaculture Practices BEMP Broadscale Environmental Monitoring Program CAMBA China-Australia Migratory Bird Agreement CEO Chief Executive Officer COBP Code of Best Practice CSER corporate, social and environmental responsibility CSIRO Commonwealth Scientific and Industrial Research Organisation DAFF Depart of Agriculture, Fisheries and Forestry dBA A-weighted decibels DMB Dry matter basis DO dissolved oxygen DPIW Department of Primary Industries and Water DPIPWE Department of Primary Industries, Parks, Water and the Environment EDO Environmental Defenders Office ENGOs environmental non-governmental organisations EIS Environmental Impact Statement EMS Environmental Management System EPA Environmental Protection Authority EPBCA Environmental Protection and Biodiversity Conservation Act 1999 FCR Feed Conversion Ratio FHMP Fish Health Management Plan FSANZ Food Standards Australia New Zealand g gram GAA Global Aquaculture Alliance ha hectare HAB Harmful Algal Bloom HOG head on gutted HVN Huon Valley News IALA International Association of Lighthouse Authorities IMAS Institute of Marine and Antarctic Studies i JAMBA Japan-Australia Migratory Bird Agreement kg kilogram km kilometre L litre LED light-emitting diode m metre mm millimetre MAST Marine and Safety -

First Images in the Wild of Blackthroat Luscinia Obscura, Asia's Most
BirdingASIA 15 (2011): 17–19 17 LITTLE-KNOWN ASIAN BIRD First images in the wild of Blackthroat Luscinia obscura, Asia’s most enigmatic robin WEI QIAN & HE YI Background probably a female from Baihe Nature Reserve, The Blackthroat Luscinia obscura is known to breed Sichuan, on 3 June 2007 (Anderson 2007). only in the mountains of south-west China, where Additionally, a few captive birds, assumed to have there are a few scattered records from Sichuan, been caught in the vicinity of Chengdu, have Gansu and Shaanxi, together with even fewer appeared in Chengdu bird market (Wang 2004). presumed non-breeding records from Yunnan All other records are from Thailand and presumed (southern China) and northern Thailand. It is an to be either wintering birds or passage migrants extremely poorly known species classified as (BirdLife International 2001). Vulnerable because it is inferred to have a small, declining population as a result of destruction of Observations temperate forest within its breeding area as well On the morning of 2 May 2011, we visited Sichuan as habitat loss in its likely wintering areas (BirdLife University to observe and photograph migrating International 2001, 2011). birds in a small patch of wood and shrubbery in Since its description in 1891, there have only the south-eastern corner of the campus. At about been nine records from China, including some 09h20 a bird abruptly flew into the shrubbery, uncertain ones. These records comprise one each which we quickly located and identified as a male from Ganshu and Shaanxi, two from Yunnan, Blackthroat Luscinia obscura. We continued to presumed to be during migration, and four from observe it until about 17h20 when we left and Sichuan, all detailed in BirdLife International obtained what we believe to be the first images of (2001), and a recent sight record of a male and this species in the wild (Plates 1 & 2). -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -
![The Song Structure of the Siberian Blue Robin Luscinia [Larvivora] Cyane and a Comparison with Related Species](https://docslib.b-cdn.net/cover/7826/the-song-structure-of-the-siberian-blue-robin-luscinia-larvivora-cyane-and-a-comparison-with-related-species-897826.webp)
The Song Structure of the Siberian Blue Robin Luscinia [Larvivora] Cyane and a Comparison with Related Species
Ornithol Sci 16: 71 – 77 (2017) ORIGINAL ARTICLE The song structure of the Siberian Blue Robin Luscinia [Larvivora] cyane and a comparison with related species Vladimir IVANITSKII1,#, Alexandra IVLIEVA2, Sergey GASHKOV3 and Irina MAROVA1 1 Lomonosov Moscow State University, Biology, 119899 Moscow Leninskie Gory, Moscow, Moscow 119899, Russian Federation 2 M.F. Vladimirskii Moscow Regional Research Clinical Institute, Moscow, Moscow, Russian Federation 3 Tomsk State University-Zoology Museum, Tomsk, Tomsk, Russian Federation ORNITHOLOGICAL Abstract We studied the song syntax of the Siberian Blue Robin Luscinia cyane, a small insectivorous passerine of the taiga forests of Siberia and the Far East. Males SCIENCE have repertoires of 7 to 14 (mean 10.9±2.3) song types. A single song typically con- © The Ornithological Society sists of a short trill comprised of from three to six identical syllables, each of two to of Japan 2017 three notes; sometimes the trill is preceded by a short single note. The most complex songs contain as many as five or six different trills and single notes. The song of the Siberian Blue Robin most closely resembles that of the Indian Blue Robin L. brunnea. The individual repertoires of Siberian Blue Robin, Common Nightingale L. megarhynchos and Thrush Nightingale L. luscinia contain groups of mutually associ- ated song types that are sung usually one after another. The Siberian Blue Robin and the Common Nightingale perform them in a varying sequence, while Thrush Nightin- gale predominantly uses a fixed sequence of song types. The distinctions between the song syntax of Larvivora spp. and Luscinia spp. are discussed. -

Conserving Critically Endangered Spotted Handfish
Conserving Critically Endangered spotted handfish Unique and quirky, spotted handfish (Brachionichthys hirsutus) are recognisable by their modified fins that resemble human hands. Once common in southern Tasmania’s Derwent estuary, spotted handfish experienced a severe decline in the 1980s. In 1996 they became the first marine fish to be listed as Critically Endangered by the IUCN Red List of Threatened Species. They are also listed as Critically Endangered under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999, and Endangered under Tasmania's Threatened Species Protection Act 1995. Spotted handfish were once common in Tasmania’s Derwent estuary, but their populations experienced CSIRO, University of Tasmania (UTAS), the a serious decline in the 1980s. Tasmanian and Australian governments and the Derwent Estuary Program (DEP) have between 5–15m. Within bays they occupy been working together to conserve spotted habitats with more complex features such as handfish since the mid-1990s. depressions in the seabed made by stingrays, or fields of sea-squirts. Distribution Lacking swim bladders, spotted handfish use Handfish belong to a group of coastal their modified fins to ‘walk’ across the seabed anglerfish with a narrow distribution in south- rather than swim. Movement studies suggest eastern Australia. There are 14 species with they only travel small distances: 10m–460m seven endemic to Tasmania and Bass Strait. over many months, or an average of 4m a day. Spotted handfish were once prevalent along Spotted handfish are ambush predators and, Tasmania’s eastern coast, and were so like their close cousins the deep-sea angler common that during the 1960s and ‘70s that fishes, they have a lure located just above the they were routinely collected for practical mouth, perhaps to entice their prey of demonstrations at Hobart’s university. -

Diversity and Risk Patterns of Freshwater Megafauna: a Global Perspective
Diversity and risk patterns of freshwater megafauna: A global perspective Inaugural-Dissertation to obtain the academic degree Doctor of Philosophy (Ph.D.) in River Science Submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin By FENGZHI HE 2019 This thesis work was conducted between October 2015 and April 2019, under the supervision of Dr. Sonja C. Jähnig (Leibniz-Institute of Freshwater Ecology and Inland Fisheries), Jun.-Prof. Dr. Christiane Zarfl (Eberhard Karls Universität Tübingen), Dr. Alex Henshaw (Queen Mary University of London) and Prof. Dr. Klement Tockner (Freie Universität Berlin and Leibniz-Institute of Freshwater Ecology and Inland Fisheries). The work was carried out at Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany, Freie Universität Berlin, Germany and Queen Mary University of London, UK. 1st Reviewer: Dr. Sonja C. Jähnig 2nd Reviewer: Prof. Dr. Klement Tockner Date of defense: 27.06. 2019 The SMART Joint Doctorate Programme Research for this thesis was conducted with the support of the Erasmus Mundus Programme, within the framework of the Erasmus Mundus Joint Doctorate (EMJD) SMART (Science for MAnagement of Rivers and their Tidal systems). EMJDs aim to foster cooperation between higher education institutions and academic staff in Europe and third countries with a view to creating centres of excellence and providing a highly skilled 21st century workforce enabled to lead social, cultural and economic developments. All EMJDs involve mandatory mobility between the universities in the consortia and lead to the award of recognised joint, double or multiple degrees. The SMART programme represents a collaboration among the University of Trento, Queen Mary University of London and Freie Universität Berlin. -

Handfish Survey in the Vicinity of a Proposed Intake Pipeline
HANDFISH SURVEY IN THE VICINITY OF A PROPOSED INTAKE PIPELINE AND EXISTING INTAKE INSPECTION AT CRAYFISH POINT, TAROONA, DERWENT ESTUARY Report to IMAS March 2018 www.marinesolutions.net.au © Marine Solutions 2018. This document should only be used for the specific project and purposes for which it was commissioned. 1 Version Author Date Reviewed by reviewed 1 of 1 Annie Ford and 20/03/2018 Laura Smith Joanna Smart Note: Location maps throughout this report are representative only; for precise GPS coordinates, see the appendices. All satellite imagery used throughout is sourced from The Land Information System Tasmania (LIST). 1 Cover photo, IMAS Taroona, November 2016 (photo by Marine Solutions). Handfish survey at site of proposed intake pipeline, Taroona 2 TABLE OF CONTENTS Table of Contents .......................................................................................................................................... 3 Table of Figures ............................................................................................................................................. 5 1 Executive Summary ............................................................................................................................... 6 2 Introduction .......................................................................................................................................... 8 2.1 Background .................................................................................................................................. -

South-East Marine Region Profile
South-east marine region profile A description of the ecosystems, conservation values and uses of the South-east Marine Region June 2015 © Commonwealth of Australia 2015 South-east marine region profile: A description of the ecosystems, conservation values and uses of the South-east Marine Region is licensed by the Commonwealth of Australia for use under a Creative Commons Attribution 3.0 Australia licence with the exception of the Coat of Arms of the Commonwealth of Australia, the logo of the agency responsible for publishing the report, content supplied by third parties, and any images depicting people. For licence conditions see: http://creativecommons.org/licenses/by/3.0/au/ This report should be attributed as ‘South-east marine region profile: A description of the ecosystems, conservation values and uses of the South-east Marine Region, Commonwealth of Australia 2015’. The Commonwealth of Australia has made all reasonable efforts to identify content supplied by third parties using the following format ‘© Copyright, [name of third party] ’. Front cover: Seamount (CSIRO) Back cover: Royal penguin colony at Finch Creek, Macquarie Island (Melinda Brouwer) B / South-east marine region profile South-east marine region profile A description of the ecosystems, conservation values and uses of the South-east Marine Region Contents Figures iv Tables iv Executive Summary 1 The marine environment of the South-east Marine Region 1 Provincial bioregions of the South-east Marine Region 2 Conservation values of the South-east Marine Region 2 Key ecological features 2 Protected species 2 Protected places 2 Human activities and the marine environment 3 1.