The Song Structure of the Siberian Blue Robin Luscinia [Larvivora] Cyane and a Comparison with Related Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Salt Exploitation and Landscape Structure in a Breeding Population of the Threatened Bluethroat (Luscinia Svecica) in Salt-Pans in Western France
Biological Conservation 107 (2002) 283–289 www.elsevier.com/locate/biocon Salt exploitation and landscape structure in a breeding population of the threatened bluethroat (Luscinia svecica) in salt-pans in western France T. Geslina,*, J.-C. Lefeuvrea, Y. Le Pajoleca, S. Questiaub, M.C. Eyberta aUMR 6553 ECOBIO, Universite´ de Rennes I, Avenue du Ge´ne´ral Leclerc, 35042 Rennes cedex, France bLaboratoire d’Ecologie animale, Universite´ d’Angers, 2 Boulevard Lavoisier, Campus Belle-Beille, 49045 Angers cedex, France Received 12 June 2001; received in revised form 10 January 2002; accepted 10 January 2002 Abstract The Gue´ rande salt-pans represent the main French breeding area of bluethroat, a migrating passerine. Salt exploitation has cre- ated a geometrical artificial landscape in which we investigated factors influencing spatial distribution and breeding success of this species using a Geographical Information System. We compared data for four sites in these salt-pans, for three zones in the most densely populated site, and for 2500 m2 grid cells defined for this same site. This study showed the influence of (1) the level of salt exploitation activity, (2) the density of bank intersections, (3) the extent of area covered by Suaeda vera bushes and (4) the structural heterogeneity. The continued management of these salt-pans enhanced bird breeding success. Thus, traditional salt exploitation contributes directly to the conservation of bluethroat, considered as an endangered species in Europe. # 2002 Elsevier Science Ltd. All rights reserved. Keywords: Breeding bluethroat; Human activity; Salt-pans area; Landscape heterogeneity; Breeding success 1. Introduction throats. Then, bluethroats decreased and finally dis- appeared with decline of salt extraction and use of The bluethroat (Luscinia svecica) is a migrating pas- banks for crops and pasture. -

Rare Birds of California Now Available! Price $54.00 for WFO Members, $59.99 for Nonmembers
Volume 40, Number 3, 2009 The 33rd Report of the California Bird Records Committee: 2007 Records Daniel S. Singer and Scott B. Terrill .........................158 Distribution, Abundance, and Survival of Nesting American Dippers Near Juneau, Alaska Mary F. Willson, Grey W. Pendleton, and Katherine M. Hocker ........................................................191 Changes in the Winter Distribution of the Rough-legged Hawk in North America Edward R. Pandolfino and Kimberly Suedkamp Wells .....................................................210 Nesting Success of California Least Terns at the Guerrero Negro Saltworks, Baja California Sur, Mexico, 2005 Antonio Gutiérrez-Aguilar, Roberto Carmona, and Andrea Cuellar ..................................... 225 NOTES Sandwich Terns on Isla Rasa, Gulf of California, Mexico Enriqueta Velarde and Marisol Tordesillas ...............................230 Curve-billed Thrasher Reproductive Success after a Wet Winter in the Sonoran Desert of Arizona Carroll D. Littlefield ............234 First North American Records of the Rufous-tailed Robin (Luscinia sibilans) Lucas H. DeCicco, Steven C. Heinl, and David W. Sonneborn ........................................................237 Book Reviews Rich Hoyer and Alan Contreras ...........................242 Featured Photo: Juvenal Plumage of the Aztec Thrush Kurt A. Radamaker .................................................................247 Front cover photo by © Bob Lewis of Berkeley, California: Dusky Warbler (Phylloscopus fuscatus), Richmond, Contra Costa County, California, 9 October 2008, discovered by Emilie Strauss. Known in North America including Alaska from over 30 records, the Dusky is the Old World Warbler most frequent in western North America south of Alaska, with 13 records from California and 2 from Baja California. Back cover “Featured Photos” by © Kurt A. Radamaker of Fountain Hills, Arizona: Aztec Thrush (Ridgwayia pinicola), re- cently fledged juvenile, Mesa del Campanero, about 20 km west of Yecora, Sonora, Mexico, 1 September 2007. -
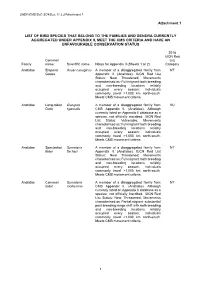
Attachment 1 LIST of BIRD SPECIES THAT BELONG to the FAMILIES
UNEP/CMS/ScC-SC4/Doc.11.3.2/Attachment 1 Attachment 1 LIST OF BIRD SPECIES THAT BELONG TO THE FAMILIES AND GENERA CURRENTLY AGGREGATED UNDER APPENDIX II, MEET THE CMS CRITERIA AND HAVE AN UNFAVOURABLE CONSERVATION STATUS 2018 IUCN Red Common List Family name Scientific name Notes for Appendix II (Sheets 1 or 2) Category Anatidae Emperor Anser canagicus A member of a disaggregated family from NT Goose Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Long-tailed Clangula A member of a disaggregated family from VU Duck hyemalis CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Vulnerable; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Spectacled Somateria A member of a disaggregated family from NT Eider fischeri Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Common Somateria A member of a disaggregated family from NT Eider mollissima CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Near Threatened; Movements characterised as: Partial migrant: substantial post-breeding range shift with both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. -

Birdlife International for the Input of Analyses, Technical Information, Advice, Ideas, Research Papers, Peer Review and Comment
UNEP/CMS/ScC16/Doc.10 Annex 2b CMS Scientific Council: Flyway Working Group Reviews Review 2: Review of Current Knowledge of Bird Flyways, Principal Knowledge Gaps and Conservation Priorities Compiled by: JEFF KIRBY Just Ecology Brookend House, Old Brookend, Berkeley, Gloucestershire, GL13 9SQ, U.K. June 2010 Acknowledgements I am grateful to colleagues at BirdLife International for the input of analyses, technical information, advice, ideas, research papers, peer review and comment. Thus, I extend my gratitude to my lead contact at the BirdLife Secretariat, Ali Stattersfield, and to Tris Allinson, Jonathan Barnard, Stuart Butchart, John Croxall, Mike Evans, Lincoln Fishpool, Richard Grimmett, Vicky Jones and Ian May. In addition, John Sherwell worked enthusiastically and efficiently to provide many key publications, at short notice, and I’m grateful to him for that. I also thank the authors of, and contributors to, Kirby et al. (2008) which was a major review of the status of migratory bird species and which laid the foundations for this work. Borja Heredia, from CMS, and Taej Mundkur, from Wetlands International, also provided much helpful advice and assistance, and were instrumental in steering the work. I wish to thank Tim Jones as well (the compiler of a parallel review of CMS instruments) for his advice, comment and technical inputs; and also Simon Delany of Wetlands International. Various members of the CMS Flyway Working Group, and other representatives from CMS, BirdLife and Wetlands International networks, responded to requests for advice and comment and for this I wish to thank: Olivier Biber, Joost Brouwer, Nicola Crockford, Carlo C. Custodio, Tim Dodman, Roger Jaensch, Jelena Kralj, Angus Middleton, Narelle Montgomery, Cristina Morales, Paul Kariuki Ndang'ang'a, Paul O’Neill, Herb Raffaele and David Stroud. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Luscinia Luscinia)
Ornis Hungarica 2018. 26(1): 149–170. DOI: 10.1515/orhu-2018-0010 Exploratory analyses of migration timing and morphometrics of the Thrush Nightingale (Luscinia luscinia) Tibor CSÖRGO˝ 1 , Péter FEHÉRVÁRI2, Zsolt KARCZA3, Péter ÓCSAI4 & Andrea HARNOS2* Received: April 20, 2018 – Revised: May 10, 2018 – Accepted: May 20, 2018 Tibor Csörgo,˝ Péter Fehérvári, Zsolt Karcza, Péter Ócsai & Andrea Harnos 2018. Exploratory analyses of migration timing and morphometrics of the Thrush Nightingale (Luscinia luscinia). – Ornis Hungarica 26(1): 149–170. DOI: 10.1515/orhu-2018-0010 Abstract Ornithological studies often rely on long-term bird ringing data sets as sources of information. However, basic descriptive statistics of raw data are rarely provided. In order to fill this gap, here we present the seventh item of a series of exploratory analyses of migration timing and body size measurements of the most frequent Passerine species at a ringing station located in Central Hungary (1984–2017). First, we give a concise description of foreign ring recoveries of the Thrush Nightingale in relation to Hungary. We then shift focus to data of 1138 ringed and 547 recaptured individuals with 1557 recaptures (several years recaptures in 76 individuals) derived from the ringing station, where birds have been trapped, handled and ringed with standardized methodology since 1984. Timing is described through annual and daily capture and recapture frequencies and their descriptive statistics. We show annual mean arrival dates within the study period and present the cumulative distributions of first captures with stopover durations. We present the distributions of wing, third primary, tail length and body mass, and the annual means of these variables. -

Thailand Highlights 14Th to 26Th November 2019 (13 Days)
Thailand Highlights 14th to 26th November 2019 (13 days) Trip Report Siamese Fireback by Forrest Rowland Trip report compiled by Tour Leader: Forrest Rowland Trip Report – RBL Thailand - Highlights 2019 2 Tour Summary Thailand has been known as a top tourist destination for quite some time. Foreigners and Ex-pats flock there for the beautiful scenery, great infrastructure, and delicious cuisine among other cultural aspects. For birders, it has recently caught up to big names like Borneo and Malaysia, in terms of respect for the avian delights it holds for visitors. Our twelve-day Highlights Tour to Thailand set out to sample a bit of the best of every major habitat type in the country, with a slight focus on the lush montane forests that hold most of the country’s specialty bird species. The tour began in Bangkok, a bustling metropolis of winding narrow roads, flyovers, towering apartment buildings, and seemingly endless people. Despite the density and throng of humanity, many of the participants on the tour were able to enjoy a Crested Goshawk flight by Forrest Rowland lovely day’s visit to the Grand Palace and historic center of Bangkok, including a fun boat ride passing by several temples. A few early arrivals also had time to bird some of the urban park settings, even picking up a species or two we did not see on the Main Tour. For most, the tour began in earnest on November 15th, with our day tour of the salt pans, mudflats, wetlands, and mangroves of the famed Pak Thale Shore bird Project, and Laem Phak Bia mangroves. -

Sichuan, China
Tropical Birding: Sichuan (China). Custom Tour Report A Tropical Birding custom tour SICHUAN, CHINA : (Including the Southern Shans Pre-tour Extension) WHITE-THROATED TIT One of 5 endemic tits recorded on the tour. 21 May – 12 June, 2010 Tour Leader: Sam Woods All photos were taken by Sam Woods/Tropical Birding on this tour, except one photo. www.tropicalbirding.com [email protected] 1-409-515-0514 Tropical Birding: Sichuan (China). Custom Trip Report The Central Chinese province of Sichuan provided some notable challenges this year: still recovering from the catastrophic “Wenchuan 5.12” earthquake of 2008, the area is undergoing massive reconstruction. All very positive for the future of this scenically extraordinary Chinese region, but often a headache for tour arrangements, due to last minute traffic controls leading us to regularly rethink our itinerary in the Wolong area in particular, that was not far from the epicenter of that massive quake. Even in areas seemingly unaffected by the quake, huge road construction projects created similar challenges to achieving our original planned itinerary. However, in spite of regular shuffling and rethinking, the itinerary went ahead pretty much as planned with ALL sites visited. Other challenges came this year in the form of heavy regular rains that plagued us at Wawu Shan and low cloud that limited visibility during our time around the breathtaking Balang Mountain in the Wolong region. With some careful trickery, sneaking our way through week-long road blocks under cover of darkness, birding through thick and thin (mist, cloud and rains) we fought against all such challenges and came out on top. -

Sri Lanka: January 2015
Tropical Birding Trip Report Sri Lanka: January 2015 A Tropical Birding CUSTOM tour SRI LANKA: Ceylon Sojourn 9th- 23rd January 2015 Tour Leaders: Sam Woods & Chaminda Dilruk SRI LANKA JUNGLEFOWL is Sri Lanka’s colorful national bird, which was ranked among the top five birds of the tour by the group. All photos in this report were taken by Sam Woods. 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report Sri Lanka: January 2015 INTRODUCTION In many ways Sri Lanka covers it all; for the serious birder, even those with experience from elsewhere in the Indian subcontinent, it offers up a healthy batch of at least 32 endemic bird species (this list continues to grow, though, so could increase further yet); for those without any previous experience of the subcontinent it offers these but, being an island of limited diversity, not the overwhelming numbers of birds, which can be intimidating for the first timer; and for those with a natural history slant that extends beyond the avian, there is plentiful other wildlife besides, to keep all happy, such as endemic monkeys, strange reptiles only found on this teardrop-shaped island, and a bounty of butterflies, which feature day-in, day-out. It should also be made clear that while it appears like a chunk of India which has dropped of the main subcontinent, to frame it, as merely an extension of India, would be a grave injustice, as Sri Lanka feels, looks, and even tastes very different. There are some cultural quirks that make India itself, sometimes challenging to visit for the westerner. -

Territoriality As a Paternity Guard in the European Robin, Erithacus Rubecula
ANIMAL BEHAVIOUR, 2000, 60, 165–173 doi:10.1006/anbe.2000.1442, available online at http://www.idealibrary.com on ARTICLES Territoriality as a paternity guard in the European robin, Erithacus rubecula JOE TOBIAS & NAT SEDDON Department of Zoology, University of Cambridge (Received 29 June 1999; initial acceptance 29 July 1999; final acceptance 18 March 2000; MS. number: 6273) To investigate the relative importance of paternity defences in the European robin we used behavioural observations, simulated intrusions and temporary male removal experiments. Given that paired males did not increase their mate attendance, copulation rate or territory size during the female’s fertile period, the most frequently quoted paternity assurance strategies in birds were absent. However, males with fertile females sang and patrolled their territories more regularly, suggesting that territorial motivation and vigilance were elevated when the risk of cuckoldry was greatest. In addition, there was a significant effect of breeding period on response to simulated intrusions: residents approached and attacked freeze-dried mounts more readily in the fertile period. During 90-min removals of the pair male in the fertile period, neighbours trespassed more frequently relative to prefertile and fertile period controls and appeared to seek copulations with unattended females. When replaced on their territories, males immediately increased both song rate and patrolling rate in comparison with controls. We propose that male robins sing to signal their presence, and increase their territorial vigilance and aggression in the fertile period to protect paternity. 2000 The Association for the Study of Animal Behaviour Despite the apparent social monogamy of the majority of Thryothorus nigricapillus: Levin 1996). -

Recent Evolutionary History of the Bluethroat (Luscinia Svecica)
Molecular Ecology (2003) 12, 3069–3075 doi: 10.1046/j.1365-294X.2003.01981.x RecentBlackwell Publishing Ltd. evolutionary history of the bluethroat (Luscinia svecica) across Eurasia ROBERT M. ZINK,* SERGEI V. DROVETSKI,† SOPHIE QUESTIAU,‡ IGOR V. FADEEV,§ EVGENIY V. NESTEROV,§ MICHAEL C. WESTBERG* and SIEVERT ROHWER¶ *J. F. Bell Museum of Natural History, University of Minnesota, St Paul, MN 55108, USA, †Department of Ecology, Evolution, and Behavior, University of Minnesota, St Paul, MN 55108, USA, ‡Ecologie Animale, Université d’Angers, Belle-Beille, 2, boulevard Lavoisier, F-49045 Angers cedex, France, §State Darwin Museum, 57 Vavilova Street, Moscow 117292, Russia, ¶Burke Museum and Department of Zoology, University of Washington, Seattle, WA 98195–3010, USA Abstract We analysed mitochondrial DNA (mtDNA) sequences from 154 bluethroats (Luscinia svecica) sampled at 21 sites throughout much of their Eurasian range. A previously reported, single base-pair mtDNA difference between L. s. svecica and L. s. namnetum was inconsistent upon expanded geographical sampling. A significant FST value (0.29) and an isolation-by-distance effect show the existence of geographical differentiation. Phyloge- netic analysis of haplotypes revealed northern and southern groups, although lineage sort- ing is incomplete. There was no geographical structure to the haplotype tree within groups, and currently recognized subspecies were not supported. A minimum evolution tree based on pairwise mtDNA genetic distances among average samples showed the same two broadly distributed northern and southern groups. These groups abut in the centre of the latitudinal range, and were possibly isolated by forest that developed and spread westward over the last 15 000 years. -

Rejection Behavior by Common Cuckoo Hosts Towards Artificial Brood Parasite Eggs
REJECTION BEHAVIOR BY COMMON CUCKOO HOSTS TOWARDS ARTIFICIAL BROOD PARASITE EGGS ARNE MOKSNES, EIVIN ROSKAFT, AND ANDERS T. BRAA Departmentof Zoology,University of Trondheim,N-7055 Dragvoll,Norway ABSTRACT.--Westudied the rejectionbehavior shown by differentNorwegian cuckoo hosts towardsartificial CommonCuckoo (Cuculus canorus) eggs. The hostswith the largestbills were graspejectors, those with medium-sizedbills were mostlypuncture ejectors, while those with the smallestbills generally desertedtheir nestswhen parasitizedexperimentally with an artificial egg. There were a few exceptionsto this general rule. Becausethe Common Cuckooand Brown-headedCowbird (Molothrus ater) lay eggsthat aresimilar in shape,volume, and eggshellthickness, and they parasitizenests of similarly sizedhost species,we support the punctureresistance hypothesis proposed to explain the adaptivevalue (or evolution)of strengthin cowbirdeggs. The primary assumptionand predictionof this hypothesisare that somehosts have bills too small to graspparasitic eggs and thereforemust puncture-eject them,and that smallerhosts do notadopt ejection behavior because of the heavycost involved in puncture-ejectingthe thick-shelledparasitic egg. We comparedour resultswith thosefor North AmericanBrown-headed Cowbird hosts and we found a significantlyhigher propor- tion of rejectersamong CommonCuckoo hosts with graspindices (i.e. bill length x bill breadth)of <200 mm2. Cuckoo hosts ejected parasitic eggs rather than acceptthem as cowbird hostsdid. Amongthe CommonCuckoo hosts, the costof acceptinga parasiticegg probably alwaysexceeds that of rejectionbecause cuckoo nestlings typically eject all hosteggs or nestlingsshortly after they hatch.Received 25 February1990, accepted 23 October1990. THEEGGS of many brood parasiteshave thick- nestseither by grasping the eggs or by punc- er shells than the eggs of other bird speciesof turing the eggs before removal. Rohwer and similar size (Lack 1968,Spaw and Rohwer 1987).