Diversity and Risk Patterns of Freshwater Megafauna: a Global Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rare Birds of California Now Available! Price $54.00 for WFO Members, $59.99 for Nonmembers
Volume 40, Number 3, 2009 The 33rd Report of the California Bird Records Committee: 2007 Records Daniel S. Singer and Scott B. Terrill .........................158 Distribution, Abundance, and Survival of Nesting American Dippers Near Juneau, Alaska Mary F. Willson, Grey W. Pendleton, and Katherine M. Hocker ........................................................191 Changes in the Winter Distribution of the Rough-legged Hawk in North America Edward R. Pandolfino and Kimberly Suedkamp Wells .....................................................210 Nesting Success of California Least Terns at the Guerrero Negro Saltworks, Baja California Sur, Mexico, 2005 Antonio Gutiérrez-Aguilar, Roberto Carmona, and Andrea Cuellar ..................................... 225 NOTES Sandwich Terns on Isla Rasa, Gulf of California, Mexico Enriqueta Velarde and Marisol Tordesillas ...............................230 Curve-billed Thrasher Reproductive Success after a Wet Winter in the Sonoran Desert of Arizona Carroll D. Littlefield ............234 First North American Records of the Rufous-tailed Robin (Luscinia sibilans) Lucas H. DeCicco, Steven C. Heinl, and David W. Sonneborn ........................................................237 Book Reviews Rich Hoyer and Alan Contreras ...........................242 Featured Photo: Juvenal Plumage of the Aztec Thrush Kurt A. Radamaker .................................................................247 Front cover photo by © Bob Lewis of Berkeley, California: Dusky Warbler (Phylloscopus fuscatus), Richmond, Contra Costa County, California, 9 October 2008, discovered by Emilie Strauss. Known in North America including Alaska from over 30 records, the Dusky is the Old World Warbler most frequent in western North America south of Alaska, with 13 records from California and 2 from Baja California. Back cover “Featured Photos” by © Kurt A. Radamaker of Fountain Hills, Arizona: Aztec Thrush (Ridgwayia pinicola), re- cently fledged juvenile, Mesa del Campanero, about 20 km west of Yecora, Sonora, Mexico, 1 September 2007. -
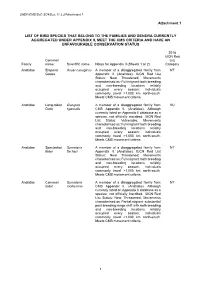
Attachment 1 LIST of BIRD SPECIES THAT BELONG to the FAMILIES
UNEP/CMS/ScC-SC4/Doc.11.3.2/Attachment 1 Attachment 1 LIST OF BIRD SPECIES THAT BELONG TO THE FAMILIES AND GENERA CURRENTLY AGGREGATED UNDER APPENDIX II, MEET THE CMS CRITERIA AND HAVE AN UNFAVOURABLE CONSERVATION STATUS 2018 IUCN Red Common List Family name Scientific name Notes for Appendix II (Sheets 1 or 2) Category Anatidae Emperor Anser canagicus A member of a disaggregated family from NT Goose Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Long-tailed Clangula A member of a disaggregated family from VU Duck hyemalis CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Vulnerable; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Spectacled Somateria A member of a disaggregated family from NT Eider fischeri Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Common Somateria A member of a disaggregated family from NT Eider mollissima CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Near Threatened; Movements characterised as: Partial migrant: substantial post-breeding range shift with both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus Idus
REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1822280 REVIEW Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus idus Mehis Rohtlaa,b, Lorenzo Vilizzic, Vladimır Kovacd, David Almeidae, Bernice Brewsterf, J. Robert Brittong, Łukasz Głowackic, Michael J. Godardh,i, Ruth Kirkf, Sarah Nienhuisj, Karin H. Olssonh,k, Jan Simonsenl, Michał E. Skora m, Saulius Stakenas_ n, Ali Serhan Tarkanc,o, Nildeniz Topo, Hugo Verreyckenp, Grzegorz ZieRbac, and Gordon H. Coppc,h,q aEstonian Marine Institute, University of Tartu, Tartu, Estonia; bInstitute of Marine Research, Austevoll Research Station, Storebø, Norway; cDepartment of Ecology and Vertebrate Zoology, Faculty of Biology and Environmental Protection, University of Lodz, Łod z, Poland; dDepartment of Ecology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia; eDepartment of Basic Medical Sciences, USP-CEU University, Madrid, Spain; fMolecular Parasitology Laboratory, School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames, Surrey, UK; gDepartment of Life and Environmental Sciences, Bournemouth University, Dorset, UK; hCentre for Environment, Fisheries & Aquaculture Science, Lowestoft, Suffolk, UK; iAECOM, Kitchener, Ontario, Canada; jOntario Ministry of Natural Resources and Forestry, Peterborough, Ontario, Canada; kDepartment of Zoology, Tel Aviv University and Inter-University Institute for Marine Sciences in Eilat, Tel Aviv, -

Charismatic Megafauna
KIDS CORNER CHARISMATIC MEGAFAUNA This document aims to teach you about megafauna. This presentation has the following structure: Slide 1 - What Are Megafauna? Slide 2 - Charismatic Megafauna Slide 3 - Megafauna Extinction Theories Slide 4 - Timeline Slide 5 - Living Megafauna Slide 6 - Extinct Australian Megafauna Slide 7 - Extinct African Megafauna Slide 8 - Case Study: Diprotodon optatum Slide 9 - Australian Curriculum Mapping KIDS CORNER CHARISMATIC MEGAFAUNA What Are Megafauna? Combining the Latin words for “large” (mega) and “animals” (fauna) creates the word “megafauna.” Megafauna are the largest animals on Earth – the ones that dominate the landscape during the time in which they live. Dinosaurs were certainly the megafauna of their time. And after the dinosaurs all died off in the mass extinction at the end of the Cretaceous period, new megafauna arose. They looked a lot like their modern descendants but were much bigger. Imagine wombats the size of a compact car, birds that stood taller than a human being, or snakes that make modern pythons look puny. Scientists consider animals that weigh more than 44 kilograms as adults to be megafauna. The term applies not only to mammals, but also to birds, reptiles, and amphibians—in short, all vertebrates, or animals with a backbone. By that definition, there are plenty of megafauna walking the Earth and swimming in its oceans today. Gorillas, elephants, and whales are prime examples. KIDS CORNER CHARISMATIC MEGAFAUNA Charismatic Megafauna The word “charismatic” means “charming” or “fascinating.” Conservationists coined the term “charismatic megafauna” during the 1980s to acknowledge that people find large animals very interesting, especially large animals that exhibit endearing or intriguing behaviour. -

Fish, Various Invertebrates
Zambezi Basin Wetlands Volume II : Chapters 7 - 11 - Contents i Back to links page CONTENTS VOLUME II Technical Reviews Page CHAPTER 7 : FRESHWATER FISHES .............................. 393 7.1 Introduction .................................................................... 393 7.2 The origin and zoogeography of Zambezian fishes ....... 393 7.3 Ichthyological regions of the Zambezi .......................... 404 7.4 Threats to biodiversity ................................................... 416 7.5 Wetlands of special interest .......................................... 432 7.6 Conservation and future directions ............................... 440 7.7 References ..................................................................... 443 TABLE 7.2: The fishes of the Zambezi River system .............. 449 APPENDIX 7.1 : Zambezi Delta Survey .................................. 461 CHAPTER 8 : FRESHWATER MOLLUSCS ................... 487 8.1 Introduction ................................................................. 487 8.2 Literature review ......................................................... 488 8.3 The Zambezi River basin ............................................ 489 8.4 The Molluscan fauna .................................................. 491 8.5 Biogeography ............................................................... 508 8.6 Biomphalaria, Bulinis and Schistosomiasis ................ 515 8.7 Conservation ................................................................ 516 8.8 Further investigations ................................................. -

Linnaeus at Home
NATURE-BASED ACTIVITIES FOR PARENTS LINNAEUS 1 AT HOME A GuiDE TO EXPLORING NATURE WITH CHILDREN Acknowledgements Written by Joe Burton Inspired by Carl Linnaeus With thanks to editors and reviewers: LINNAEUS Lyn Baber, Melissa Balzano, Jane Banham, Sarah Black, Isabelle Charmantier, Mark Chase, Maarten Christenhusz, Alex Davey, Gareth Dauley, AT HOME Zia Forrai, Jon Hale, Simon Hiscock, Alice ter Meulen, Lynn Parker, Elizabeth Rollinson, James Rosindell, Daryl Stenvoll-Wells, Ross Ziegelmeier Share your explorations @LinneanLearning #LinnaeusAtHome Facing page: Carl Linnaeus paper doll, illustrated in 1953. © Linnean Society of London 2019 All rights reserved. No part of this publication may be reproduced, stored in a retrival system or trasmitted in any form or by any means without the prior consent of the copyright owner. www.linnean.org/learning “If you do not know Introduction the names of things, the knowledge of them is Who was Carl Linnaeus? Contents Pitfall traps 5 lost too” Carl Linnaeus was one of the most influential scientists in the world, - Carl Linnaeus A bust of ‘The Young Linnaeus’ by but you might not know a lot about him. Thanks to Linnaeus, we Bug hunting 9 Anthony Smith (2007). have a naming system for all species so that we can understand how different species are related and can start to learn about the origins Plant hunting 13 of life on Earth. Pond dipping 17 As a young man, Linnaeus would study the animals, plants, Bird feeders 21 minerals and habitats around him. By watching the natural world, he began to understand that all living things are adapted to their Squirrel feeders 25 environments and that they can be grouped together by their characteristics (like animals with backbones, or plants that produce Friendly spaces 29 spores). -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version
Designation date: 23/06/99 Ramsar Site no. 999 Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. Dr. Srey Sunleang, DD MM YY Director, Department of Wetlands and Coastal Zones, Ministry of Environment, #48 Preah Sihanouk Blvd., Tonle Bassac, Designation date Site Reference Number Chamkar Morn, Phnom Penh, Cambodia Tel: (855) 77-333-456 Fax: (855)-23-721-073 E-mail: [email protected] 2. -

Overview of the Key Fish Species and Their Biology in Himalayan Rivers in Nepal Tek Bahadur Gurung, Arun Baidya, Gopal Lamsal, Nita Pradhan
Overview of the key fish species and their biology in Himalayan Rivers in Nepal Tek Bahadur Gurung, Arun Baidya, Gopal Lamsal, Nita Pradhan Regional Meeting of Fish Experts 29-30 April, 2018, Hotel Yak and Yeti Organized by Kathmandu, Nepal 1 Nepal is endowed with 232 fish species, 217 indigenous in 6000 rivers, the river basins extending to China, Nepal & India in 3 river basins & 1 river system 2 Species Richness Low High mount Moderate Mid hills Flood plains Rich Cool water fish (not permanently in cold or warm waters), most life history strategies (12 to 29oC), Cold water species (7-20oC) Warm water (15 to 32oC) 3 The Key Fish Species of Himalayan Rivers Key fish species are those : • Rare, endangered, threatened RET Species in Nepal Himalaya species as per IUCN criteria • Endemic species Endemic species reported • Exhibiting Habitat Diversity Number of species at altitudinal and migratory Pathways basis and migratory pathways • Spawning Biology Ex-situ conservation • Conservation Biology In-situ co-managing conservation Most important biotic and abiotic factors of a river • Water flow • Substrate 210 cross dam projects in different rivers • Light (NEA 2013): • Temperature • 84 in operation, • Water chemistry • 34 under construction, • Bacteria • 92 proposed • Underwater plants • Invertebrates • Fish • Birds ….. and the communities Location of Cross Dams Source: ADB 2014 Flows, Fish Species & Livelihood : Generalised Scenario et al 2016al et Gurung Source : Source 6 General features of the Himalayan Rivers • Himalayan rivers have -

Proceedings of a Workshop on the Development of a Genetic Improvement Program for African Catfish Clarias Gariepinus
Proceedings of a Workshop on the Development of a Genetic Improvement Program for African Catfish African catfish (Clarias gariepinus) production has gained considerable importance in a number of African countries. The species has several desirable attributes that make it attractive for aquaculture development. It is easy to reproduce, it does not require specialized feed, it tolerates high stocking densities, it accepts artificial feed, it tolerates poor water quality, and very importantly, it is highly sought after in local markets and economically viable in pond production systems. The species is endemic to Africa. In 2007 the WorldFish Center organized a workshop in Accra, Ghana, hosted by the Water Research Institute, to review the status of the catfish industry in Africa and develop recommendations on how best to approach the issue of genetic improvement programs. The results of the workshop are presented in this volume. PROCEEDINGS | 1889 Clarias gariepinus Proceedings of a Workshop on the Development of a Genetic Improvement Program ISBN 978-983-2346-68-5 for African CatfishClarias 2008 The WorldFish Center gariepinus For further information on publications please contact: Business Development and Communications Division The WorldFish Center Edited by R.W. Ponzoni and N.H. Nguyen PO Box 500 GPO, 10670 Penang, Malaysia Tel : (+60-4) 626 1606 Fax : (+60-4) 626 5530 Email : [email protected] This publication is also available from: www.worldfishcenter.org Printed on 100% recycled paper Printed on 100% recycled Reducing poverty and hunger by improving fisheries and aquaculture www.worldfishcenter.org Proceedings of a Workshop on the Development of a Genetic Improvement Program for African Catfi sh Clarias gariepinus Accra, Ghana, 5-9 November 2007 Edited by Raul W. -

The Impact of Climate Change on a Tropical Carnivore: from Individual to Species
The Impact of Climate Change on a Tropical Carnivore: From Individual to Species Daniella Dakin Rabaiotti A dissertation submitted for the degree of Doctor of Philosophy UCL 2 Declaration I, Daniella Rabaiotti, confirm the work presented in this thesis is my own. The research was supported by NERC through the London NERC DTP. All data analysis data visualisation and modelling was done by Daniella Rabaiotti. Tim Coulson provided training in individual based modelling. Mike Croucher assisted in code optimisation for the individual based model. All chapters of this thesis were written by Daniella Rabaiotti, with guidance and comments from Rosie Woodroffe and Richard Pearson. Tim Coulson provided comments on Chapter 4, and Rosemary Groom, J.W. McNutt and Jessica Watermeyer provided comments on Chapter 3. Data from Laikipia, Kenya, on wild dog survival and movements were collected by Rosie Woodroffe and the Kenya Rangelands Wild Dog and Cheetah Project. Data on wild dog mortality in Savé Valley, Zimbabwe were collected by Rosemary Groom and the Savé Valley team at the African Wildlife Conservation Fund. Data on wild dog mortality in the Okavango, Botswana, were collected by J.W. McNutt and the Botswana Predator Conservation Trust. Data on wild dog distributions were provided by the Rangewide Conservation Programme for Cheetah and African Wild Dogs. Cover art was designed by Daniella Rabaiotti and created by Selina Betts. Graphical abstracts were designed by Daniella Rabaiotti and Gaius J. Augustus and created by Gaius J. Augustus. 3 4 Abstract Climate change is impacting species globally. Predicting which species will be impacted, where, when, and by how much, is vital to conserve biodiversity in a warming world. -

Download (2MB)
UNIVERSITI PUTRA MALAYSIA ISOLATION, CHARACTERIZATION AND PATHOGENICITY OF EPIZOOTIC ULCERATIVE SYNDROME-RELATED Aphanomyces TOWARD AN IMPROVED DIAGNOSTIC TECHNIQUE SEYEDEH FATEMEH AFZALI FPV 2014 7 ISOLATION, CHARACTERIZATION AND PATHOGENICITY OF EPIZOOTIC ULCERATIVE SYNDROME-RELATED Aphanomyces TOWARD AN IMPROVED DIAGNOSTIC TECHNIQUE UPM By SEYEDEH FATEMEH AFZALI COPYRIGHT © Thesis Submitted to the School of Graduate Study, Universiti Putra Malaysia, in Fulfillment of the Requirement for the Degree of Doctor of Philosophy August 2014 i All material contained within the thesis, including without limitation text, logos, icons, photographs and all other artwork, is copyright material of Universiti Putra Malaysia unless otherwise stated. Use may be made of any material contained within the thesis for non-commercial purposes from the copyright holder. Commercial use of material may only be made with the express, prior, written permission of Universiti Putra Malaysia. Copyright © Universiti Putra Malaysia UPM COPYRIGHT © ii DEDICATION This dissertation is lovingly dedicated to my kind family. A special feeling of gratitude to my great parents who inspired my life through their gritty strength, enduring faith, and boundless love for family. My nice sisters and brother have never left my side and have supported me throughout the process. I also dedicate this work and give special thanks to my best friend “Hasti” for being there for me throughout the entire doctorate program. UPM COPYRIGHT © iii Abstract of thesis presented to the Senate of Universiti Putra Malaysia in fulfillment of the requirement for the degree of Doctor of Philosophy ISOLATION, CHARACTERIZATION AND PATHOGENICITY OF EPIZOOTIC ULCERATIVE SYNDROME-RELATED Aphanomyces TOWARD AN IMPROVED DIAGNOSTIC TECHNIQUE By SEYEDEH FATEMEH AFZALI August 2014 Chair: Associate Professor Hassan Hj Mohd Daud, PhD Faculty: Veterinary Medicine Epizootic ulcerative syndrome (EUS) is a seasonal and severely damaging disease in wild and farmed freshwater and estuarine fishes.