JOINT H... Pbllullon STUDY Sf. Clalr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Persons with Disabilities Directory
SARNIA-LAMBTON DIRECTORY FOR PERSONS WITH DISABILITIES | 2012-2013 INTRODUCTION The Sarnia Lambton Workforce Development Board is pleased to produce this directory of services for Persons with Disabilities in Lambton County. During the course of this project, all known services were contacted and requested to provide information about their programs and organizations. Every effort has been made to ensure that the information contained in this directory is accurate and as current as possible. If you have any information that you would like included in any future editions of this directory please submit them in writing to [email protected] The Directory can be accessed by CD-Rom or at the Sarnia Lambton Workforce Development Board website www.slwdb.org Suite 504-265 N. Front Street Sarnia, Ontario N7T 7X1 Telephone: 519-332-0000 Fax: 519-336-5822 Email: [email protected] Website: www.slwdb.org Sarnia Lambton Workforce Development Board is funded by Employment Ontario: The views expressed in this document do not reflect those of Employment Ontario. Directory for Persons with Disabilities I TABLE OF CONTENTS Introduction .............................................................................................I Emergency & Crisis Numbers .............................................................III Symbols .....................................................................................................IV SECTION 1 Equipment ............................................................................................1 -

Return to CHEMICAL VALLEY2019 Contents BACKGROUND
Ten years after Ecojustice’s report on one of Canada’s most polluted communities Return to CHEMICAL VALLEY2019 Contents BACKGROUND ....................................................................................................................................................................................................................3 Advocating for a right to a healthy and ecologically balanced environment in Chemical Valley ........................................4 THE LAND AND PEOPLE .............................................................................................................................................................................................5 INDUSTRIAL AIR POLLUTION EMISSIONS .................................................................................................................................................6 Source of the data: The National Pollutant Release Inventory (NPRI) ...............................................................................................6 2016 Air Pollution Emissions Data ............................................................................................................................................................................7 Analysis of criteria air contaminants emissions ...............................................................................................................................................8 Analysis of toxic volatile organic compounds emissions – Benzene and 1,3-Butadiene........................................................10 -

Proquest Dissertations
ASSESSMENT OF CHANGES IN PRECIPITATION INTENSITIES IN ONTARIO A Thesis Presented to The Faculty of Graduate Studies of The University of Guelph by BRANISLAVA VASIUEVIC In partial fulfilment of requirements for the degree of Master of Science December, 2007 © Branislava Vasiljevic, 2007 Library and Bibliotheque et 1*1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-36577-9 Our file Notre reference ISBN: 978-0-494-36577-9 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a la Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par Nnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. this thesis. Neither the thesis Ni la these ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent etre imprimes ou autrement may be printed or otherwise reproduits sans son autorisation. -

C.23 - Cw Info
APRIL 14, 2015 Page 1 of 93 11. C.23 - CW INFO FOR IMMEDIATE RELEASE March 25, 2015 Pettapiece presents Network Southwest plan to transport minister (Queen’s Park) – When it comes to improved local transportation options, Perth-Wellington MPP Randy Pettapiece wants to get the province on board. Today in the legislature, Pettapiece presented Steven Del Duca, Ontario’s transportation minister, with a copy of the Network Southwest plan unveiled March 18 in St. Marys. “I explained to the minister how important this is to many in our community,” said Pettapiece. “He was very receptive,” he added. The MPP also wrote to the minister on behalf of the 86 people who signed postcards to support the Network Southwest plan. He presented all the postcards directly to the minister along with a full copy of the plan. The postcards state: “High quality intercity transportation, based on rail and bus, is a necessity of any modern nation. Southwestern Ontario has a particular need, due to high road congestion, population density and diverse economic activity.” They also call for a definitive study on the concept plan as outlined by Network Southwest. Pettapiece has written and spoken many times about the importance of improved transportation options – and, in particular, the need to extend GO transit service through Perth-Wellington. Last year the MPP took the extra step of submitting an Order Paper question on the Premier’s stated intention of extending GO transit service to our riding. Pettapiece was not pleased by the response, which made no mention of rural transportation challenges, focusing instead on the government’s promises for the Greater Toronto-Hamilton area. -

Pital. We Under Stand That He Is Now Better and Up
We were also sorry to hear that Mr. Joseph Julien. chairman of the Sarnia station of the Ontario Mission, has been ill and confined to the hospital. We under stand that he is now better and up. We are not certain that he has returne4 to work. Our sympathies are. also extended to Mr. Julien on the loss of his father during the time he was confined to the hos pital. · Note to the folks in the 5<>-50 Club-we are planning a trip· to the F~rd Oakville plant in the near future. We ·will see the Ford being assembled from beginning to end and who knows there might even be a free sample or twc (ma.ke mine a Monarch please). We will have the full details concerning the date and time etc. in the near futlire. Also, plans are going ahead for a Buffet Banquet-likewise--details available soon. Join us 'n Friday evenings and get in on these interesting events. Mr. Bob Boese taught the Sunday School class this past Sun~ and from all reports the lesson was en joJ8d by all who attended. f1ig1 ~ -..PAS-c-.T,...OR-.'.-.5 STUDY -C~iP -CAl{P From t he 3tudy: The young people found out on the retreat that there is so much we can get along without. We may not always like it,but it is possible. Flashlights and gas lights, replaced the electric lights. The hard bumpy ground ~eplaced the soft mattress of. home, for the boys in the tents. Rather than the water coming to you. -
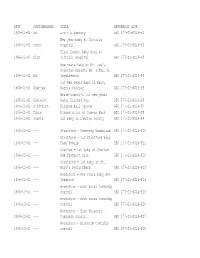
DATE PHOTOGRAPHER TITLE REFERENCE CODE 1966-01-01 Ed. Levee a Armoury AFC 177-S1-SS14-F1 1966-01-01 Chute New Year Baby at Victo
DATE PHOTOGRAPHER TITLE REFERENCE CODE 1966-01-01 Ed. Levee a Armoury AFC 177-S1-SS14-F1 New year baby at Victoria 1966-01-01 Chute Hospital AFC 177-S1-SS14-F2 First London baby born at 1966-01-01 Dick Victoria Hospital AFC 177-S1-SS14-F3 New Years baby at St. Joe's hospital parents Mr. & Mrs. B. 1966-01-01 Ed. Szymkiewicz AFC 177-S1-SS14-F4 1st New Years baby in Kent, 1966-01-01 Chatham Barrie Crozier AFC 177-S1-SS14-F5 Bruce county's 1st new years 1966-01-01 Cantelon baby, Fischer boy AFC 177-S1-SS14-F6 1966-01-01 Stratford Kingdom Hall opened AFC 177-S1-SS14-F7 1966-01-01 Chute Presentation at Queens Park AFC 177-S1-SS14-F8 1966-02-01 Sarnia 1st baby in Lambton county AFC 177-S1-SS14-F9 1966-02-01 --- Stratford - Cemetery Vandalism AFC 177-S1-SS14-F10 Stratford - 1st Stratford baby 1966-02-01 --- Faye Fowlie AFC 177-S1-SS14-F11 Chatham - 1st baby at Chatham 1966-02-01 --- PGH Thompson girl AFC 177-S1-SS14-F12 Stratford - 1st baby at St. 1966-02-01 --- Mary's David Sharp AFC 177-S1-SS14-F13 Woodstock - New Years baby Ann 1966-02-01 --- Crawford AFC 177-S1-SS14-F14 Woodstock - East Zorra township 1966-03-01 --- council AFC 177-S1-SS14-F15 Woodstock - West Zorra township 1966-03-01 --- council AFC 177-S1-SS14-F16 Woodstock - East Nissouri 1966-03-01 --- township council AFC 177-S1-SS14-F17 Woodstock - Blenheim township 1966-03-01 --- council AFC 177-S1-SS14-F18 1966-03-01 --- Chatham council inaugural AFC 177-S1-SS14-F19 Thames Secondary school, 1966-03-01 Lee opening day activities AFC 177-S1-SS14-F20 Sarnia - CIL construction at 1966-03-01 --- Courtright AFC 177-S1-SS14-F21 1966-03-01 --- Sarnia - Old hotel at Sombra AFC 177-S1-SS14-F22 Woodstock - North Oxford 1966-03-01 --- township council AFC 177-S1-SS14-F23 Sarnia - Demolition of salt 1966-03-01 --- plant AFC 177-S1-SS14-F24 St. -

Sustainable Mobility Report 2017 Working Together Towards a Sustainable Future 2017 Highlights
SUSTAINABLE MOBILITY REPORT 2017 WORKING TOGETHER TOWARDS A SUSTAINABLE FUTURE 2017 HIGHLIGHTS INCREASED NUMBER OF PASSENGER TRIPS 4.39 million total trips taken, with a 11% increase in ridership within the Quebec City – Windsor corridor, reaching 4.39 million trips INCREASED INTER-MODAL RIDERSHIP +97% increase in passenger volume WORKING from inter-modality since 2012 REDUCTION IN GHG EMISSIONS TOGETHER -34% reduction in greenhouse gas TOWARDS A SUSTAINABLE FUTURE emissions per passenger-kilometre since 2005 as a result of various energy efficiency and reduction initiatives At VIA Rail, we are playing our part in shaping the future of mobility. INVESTMENT IN SAFETY Our vision is to be the smarter way to move people by making cities AND EFFICIENCY and communities more accessible, connected and sustainable. To make our vision a reality, we must work together – with the $88.4M including capital investments in government, cities, and the private sector as well as our rail industry the fleet, equipment and major partners and the public – to create an integrated transportation infrastructure projects system that promotes Canada’s economic prosperity, enables healthier IMPROVEMENT IN LOWERING communities and makes mobility greener and more efficient. TRAIN INCIDENTS -77% reduction in our train incident ratio per million train-miles since 2014 as a result of our strengthened safety culture INCREASED AVERAGE HOURS OF TRAINING CONTENTS 02 In Conversation with the President 14 Providing the Best Customer Experience 58 Data Summary Table 46 hours -

Sarnia Streets Project, with His 1988 Publication the Origin of Sarnia’S Street Names
The Streets of Sarnia Project What’s in a (Street) Name? Randy Evans Tom St. Amand 2 Table of Contents 1. Foreword Page 4 2. Authors’ Introduction and List of Contributors Page 5 3. Preface Page 11 ● Significant Dates in Sarnia’s History ● The Naming of Sarnia’s Streets ● After Whom or What are Sarnia’s Streets Named? ● Street Designations 4. Explanations of Sarnia’s Street Names Page 25 5. Appendices Page 335 ● The Origin of the Term “Bluewater” ● Andover Lane ● Echo Road ● Egmond Drive ● Everest Court ● Grace Avenue ● Berkley Road ● Blackwell Road ● Cathcart Boulevard ● Cull Drain ● Dora Street ● Hay Street ● Livingston Street ( Pt. Edward ) ● Maria Street 3 ● McMillen Parkway ● Mitton Street ● Proctor Street ● Road Scholars ● Russell Street ● Sarnia’s Suburbs – Maxwell – The Original Bright’s Grove ● Sarnia’s Suburbs – Robertsville ● Sarnia Suburbs - City Neighbourhoods ● Talfourd Street ● The Telfer Brickyards ● Vidal Street ● Who Gets to Name Sarnia Streets? ● Woodrowe Avenue ● Wood: Its Importance to Sarnia’s Development 6. Sources / Works Cited Page 347 4 MIKE BRADLEY MAYOR CITY OF SARNIA 255 Christina Street North PO Box 3018 Sarnia, ON Canada N7T 7N2 519 – 332-0330 Ext. 3312 519-332-3995 (fax) 519 – 332-2664 (TTY) www.sarnia.ca [email protected] September 19, 2016 Dear Friends: What a wonderful journey is the Sarnia Street Project. It explores Sarnia’s rich and diverse history going back to the founding of Port Sarnia in 1836. Street names provide a fascinating look into the community’s history, diversity, culture and social and economic evolution over time. The project, while reflecting our past, also mirrors the present with hundreds of citizens contributing to the research with their own personal knowledge. -
The Streets of Sarnia Project What’S in a (Street) Name?
1 The Streets of Sarnia Project What’s in a (Street) Name? Randy Evans Tom St. Amand 2 Table of Contents 1. Foreword Page 4 2. Authors’ Introduction and List of Contributors Page 5 3. Preface Page 12 Significant Dates in Sarnia’s History The Naming of Sarnia’s Streets After Whom or What are Sarnia’s Streets Named? Street Designations 4. Explanations of Sarnia’s Street Names Page 31 5. Appendices Page 471 The Origin of the Term “Bluewater” Andover Lane Echo Road Egmond Drive Everest Court Grace Avenue Berkley Road 3 Blackwell Road Cathcart Boulevard Cull Drain Dora Street Hay Street Livingston Street ( Pt. Edward ) McMillen Parkway Proctor Street Road Scholars Russell Street Sarnia’s Suburbs – Maxwell – The Original Bright’s Grove Sarnia’s Suburbs – Robertsville Sarnia Suburbs - City Neighbourhoods Talfourd Street The Telfer Brickyards Vidal Street Who Gets to Name Sarnia Streets? Woodrowe Avenue 6. Sources / Works Cited Page 486 4 MIKE BRADLEY MAYOR CITY OF SARNIA 255 Christina Street North PO Box 3018 Sarnia, ON Canada N7T 7N2 519 – 332-0330 Ext. 3312 519-332-3995 (fax) 519 – 332-2664 (TTY) www.sarnia.ca [email protected] September 19, 2016 Dear Friends: What a wonderful journey is the Sarnia Street Project. It explores Sarnia’s rich and diverse history going back to the founding of Port Sarnia in 1836. Street names provide a fascinating look into the community’s history, diversity, culture and social and economic evolution over time. The project, while reflecting our past, also mirrors the present with hundreds of citizens contributing to the research with their own personal knowledge. -

RAIL SAFETY WEEK April 25 – May 1 2016 Calendar of Events for CANADA
RAIL SAFETY WEEK April 25 – May 1 2016 Calendar of Events for CANADA Province City/Town Event Date Event Time Event Details Name of Location and Address CHAMPLAIN REGION Safety Pledge with CN Police, Central Station - 895 rue de la Gauchetiere Quebec Montreal Monday April 25 3:30 PM TO 6:30 PM VIA, AMT and OL ** St. W H3B 2M9 Safety Pledge Commuter Gare Via Rail a Sainte-Foy 3255 chemin de Quebec Quebec Monday April 25 11:30 AM TO 1:00 PM Station Via Rail la Gare, Qc, G1W 3A8 Rail Safety Presentation - Band First Nation -Wemotachi, Rue Kenosi, Quebec Wemotachi April 25 & 26 7:30 AM TO 3:30 PM Council & Local elementary Wemotaci, QC G0X 3R0 school Rail Safety Pledge with CN Quebec Joliette Tuesday April 26 11:00 AM TO 1:00 PM 380 Rue Champlain, Joliette, QC J6E 2S1 Police, SQ Pointe-aux Quebec Tuesday April 26 4:00 PM TO 6:00 PM JVO: CN Police, AMT Pointe-aux-Trembles Station Trembles Rail Safety Presentation – First Nation –Mashteuiatsh, 525, rue Quebec Mashteuiatsh April 27 & 28 7:30 AM TO 3:30 PM Elementary and High School Uapileu, Mashteuiatsh, G0W 2H0 Quebec Monteregie Wednesday April 27 7:00 AM TO 9:00 AM Rail Safety Pledge 555, rue Boileau, Vaudreuil, Qc, J7V 9B9 4500 Hickmore et entrée 2800 Norman, Quebec Montreal Wednesday April 27 4:00 PM TO 6:00 PM Rail Safety Pledge Montreal, Qc, H4T 1K2 Rail Safety Pledge with CN Gare ViaRail 263 Rue Lindsay, Quebec Drummondville Wednesday April 27 1:00 PM TO 3:00 PM Police, SQ Drummondville, QC J2C 1P2 Rail Safety Presentation – Band First Nation –Wolinak, 10120 Rue Kolipaio, Quebec Wolinak -

Paul Edward Mikan 4193278
R15569 COLLECTION: PAUL EDWARD MIKAN 4193278 SERIES: MAPS MIKAN 4193346 Finding Aids Instrument de recherche TABLE OF CONTENTS TABLE DES MATIÈRES PART 1: ROLLED MAPS p. 2 Part 1a: Property plans p. 2 Part 1b: Right of Way plans p. 68 Part 1c: Plans which cannot be unquestionnably assigned to either one of the two p. 147 preceeding categories PART 2: "BLACK BINDERS" LAND TITLE PLANS p. 149 PART 3: INDEX CARDS TO LAND TITLES p. 159 Part 3a: Lands along former GTR Lines p. 159 (numeric index) Part 3b: Lands along former CNR Lines p. 160 (numeric index) Part 3c: Lands along former GTR Lines p. 161 (indexed by subdivision name) Part 3d Lands along former CNR Lines p. 164 (indexed by subdivision name) Part 3e: Lands along other lines owned by CNR and index cards out of sequence p. 166 COLLECTION PAUL EDWARD 1 / 166 PART 1: ROLLED MAPS Part 1a PROPERTY PLANS (sorted by original tag number) Vol. Tag No. Title of map Date Statement of responsibility Medium condition No d'étiquette Titre de la carte Mention de responsabilité Support 208 pp. 28 Plan shewing land required for drainage area & athletic grounds. Nakina. 1923-09-09 Toronto, E. Fitzgerald, O.L.S. Blueprint. Fair, adhesive tape. Unsurveyed territory. District of Thunder Bay. 257 pp. 34 Plan shewing land required for water supply at Nakina. Mile 19 west of Grant 1924-01-16 Toronto, Land Surveys Dept. Linen. Good. in unsurveyed territory. District of Thunder Bay. 287 pp. 39 (plan 1) Plan shewing property owned by the C.N.R. -

VIA Rail Looks to the Future 47 Management Discussion and Analysis 16 Review of Operations 66 Financial Statements 26 Safety 118 Corporate Directory
ANNUAL REPORT 2016 CONTENTS 02 Our Business at-a-Glance 28 Awards 04 Message from the Lead Director of the Board 32 Sustainable Mobility Report 06 Message from the President 35 Environment 08 Year at a Glance 36 Key Performance Indicators 10 2016 Milestones 38 Governance and Accountability 12 VIA Rail Looks to the Future 47 Management Discussion and Analysis 16 Review of Operations 66 Financial Statements 26 Safety 118 Corporate Directory OUR BUSINESS VIA RAIL AT-A-GLANCE WHO WE ARE VIA Rail operates Canada’s national passenger rail service on behalf of the Government of Canada. An independent Crown corporation established in 1977, VIA Rail provides a safe, cost-effective and environmentally responsible service from coast to coast in both official languages. The corporation operates close to 494 train departures weekly on a 12,500 km network, connecting over 400 Canadian communities. With approximately 2,700 active employees, VIA Rail carried 3.97 million passengers in 2016. WHERE WE OPERATE PASSENGER REVENUES PER TRAIN ROUTE 77 % Inter-City Travel 21 % Long-Distance 2 % Regional 77 % of passenger revenues are from inter-city travel (in the Corridor) PASSENGER TRIPS PER TRAIN ROUTE 94 % Inter-City Travel 4 % Long-Distance 2 % Regional Inter-City Travel Long-Distance Regional 94 % INTER-CITY TRAVEL LONG-DISTANCE REGIONAL of passenger trips consist of inter-city travel (THE CORRIDOR) TRAVEL AND TOURISM SERVICES (in the Corridor) In the densely populated In Western and Eastern VIA Rail provides passenger corridor between Québec Canada, VIA Rail’s trains service in several rural and COMMUNITIES SERVED City, QC and Windsor, ON, attract travellers from around remote regions of Canada.