A PDF Can Be Downloaded Here
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

After the Treaties: a Social, Economic and Demographic History of Maroon Society in Jamaica, 1739-1842
University of Southampton Research Repository Copyright © and Moral Rights for this thesis and, where applicable, any accompanying data are retained by the author and/or other copyright owners. A copy can be downloaded for personal non‐commercial research or study, without prior permission or charge. This thesis and the accompanying data cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content of the thesis and accompanying research data (where applicable) must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holder/s. When referring to this thesis and any accompanying data, full bibliographic details must be given, e.g. Thesis: Author (Year of Submission) "Full thesis title", University of Southampton, name of the University Faculty or School or Department, PhD Thesis, pagination. University of Southampton Department of History After the Treaties: A Social, Economic and Demographic History of Maroon Society in Jamaica, 1739-1842 Michael Sivapragasam A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in History June 2018 i ii UNIVERSITY OF SOUTHAMPTON ABSTRACT DEPARTMENT OF HISTORY Doctor of Philosophy After the Treaties: A Social, Economic and Demographic History of Maroon Society in Jamaica, 1739-1842 Michael Sivapragasam This study is built on an investigation of a large number of archival sources, but in particular the Journals and Votes of the House of the Assembly of Jamaica, drawn from resources in Britain and Jamaica. Using data drawn from these primary sources, I assess how the Maroons of Jamaica forged an identity for themselves in the century under slavery following the peace treaties of 1739 and 1740. -

A Guide to the Birds of Barrow Island
A Guide to the Birds of Barrow Island Operated by Chevron Australia This document has been printed by a Sustainable Green Printer on stock that is certified carbon in joint venture with neutral and is Forestry Stewardship Council (FSC) mix certified, ensuring fibres are sourced from certified and well managed forests. The stock 55% recycled (30% pre consumer, 25% post- Cert no. L2/0011.2010 consumer) and has an ISO 14001 Environmental Certification. ISBN 978-0-9871120-1-9 Gorgon Project Osaka Gas | Tokyo Gas | Chubu Electric Power Chevron’s Policy on Working in Sensitive Areas Protecting the safety and health of people and the environment is a Chevron core value. About the Authors Therefore, we: • Strive to design our facilities and conduct our operations to avoid adverse impacts to human health and to operate in an environmentally sound, reliable and Dr Dorian Moro efficient manner. • Conduct our operations responsibly in all areas, including environments with sensitive Dorian Moro works for Chevron Australia as the Terrestrial Ecologist biological characteristics. in the Australasia Strategic Business Unit. His Bachelor of Science Chevron strives to avoid or reduce significant risks and impacts our projects and (Hons) studies at La Trobe University (Victoria), focused on small operations may pose to sensitive species, habitats and ecosystems. This means that we: mammal communities in coastal areas of Victoria. His PhD (University • Integrate biodiversity into our business decision-making and management through our of Western Australia) -
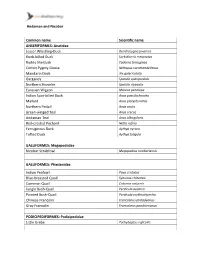
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

Seacare Authority Exemption
EXEMPTION 1—SCHEDULE 1 Official IMO Year of Ship Name Length Type Number Number Completion 1 GIANT LEAP 861091 13.30 2013 Yacht 1209 856291 35.11 1996 Barge 2 DREAM 860926 11.97 2007 Catamaran 2 ITCHY FEET 862427 12.58 2019 Catamaran 2 LITTLE MISSES 862893 11.55 2000 857725 30.75 1988 Passenger vessel 2001 852712 8702783 30.45 1986 Ferry 2ABREAST 859329 10.00 1990 Catamaran Pleasure Yacht 2GETHER II 859399 13.10 2008 Catamaran Pleasure Yacht 2-KAN 853537 16.10 1989 Launch 2ND HOME 856480 10.90 1996 Launch 2XS 859949 14.25 2002 Catamaran 34 SOUTH 857212 24.33 2002 Fishing 35 TONNER 861075 9714135 32.50 2014 Barge 38 SOUTH 861432 11.55 1999 Catamaran 55 NORD 860974 14.24 1990 Pleasure craft 79 199188 9.54 1935 Yacht 82 YACHT 860131 26.00 2004 Motor Yacht 83 862656 52.50 1999 Work Boat 84 862655 52.50 2000 Work Boat A BIT OF ATTITUDE 859982 16.20 2010 Yacht A COCONUT 862582 13.10 1988 Yacht A L ROBB 859526 23.95 2010 Ferry A MORNING SONG 862292 13.09 2003 Pleasure craft A P RECOVERY 857439 51.50 1977 Crane/derrick barge A QUOLL 856542 11.00 1998 Yacht A ROOM WITH A VIEW 855032 16.02 1994 Pleasure A SOJOURN 861968 15.32 2008 Pleasure craft A VOS SANTE 858856 13.00 2003 Catamaran Pleasure Yacht A Y BALAMARA 343939 9.91 1969 Yacht A.L.S.T. JAMAEKA PEARL 854831 15.24 1972 Yacht A.M.S. 1808 862294 54.86 2018 Barge A.M.S. -

Biodiversity: the UK Overseas Territories. Peterborough, Joint Nature Conservation Committee
Biodiversity: the UK Overseas Territories Compiled by S. Oldfield Edited by D. Procter and L.V. Fleming ISBN: 1 86107 502 2 © Copyright Joint Nature Conservation Committee 1999 Illustrations and layout by Barry Larking Cover design Tracey Weeks Printed by CLE Citation. Procter, D., & Fleming, L.V., eds. 1999. Biodiversity: the UK Overseas Territories. Peterborough, Joint Nature Conservation Committee. Disclaimer: reference to legislation and convention texts in this document are correct to the best of our knowledge but must not be taken to infer definitive legal obligation. Cover photographs Front cover: Top right: Southern rockhopper penguin Eudyptes chrysocome chrysocome (Richard White/JNCC). The world’s largest concentrations of southern rockhopper penguin are found on the Falkland Islands. Centre left: Down Rope, Pitcairn Island, South Pacific (Deborah Procter/JNCC). The introduced rat population of Pitcairn Island has successfully been eradicated in a programme funded by the UK Government. Centre right: Male Anegada rock iguana Cyclura pinguis (Glen Gerber/FFI). The Anegada rock iguana has been the subject of a successful breeding and re-introduction programme funded by FCO and FFI in collaboration with the National Parks Trust of the British Virgin Islands. Back cover: Black-browed albatross Diomedea melanophris (Richard White/JNCC). Of the global breeding population of black-browed albatross, 80 % is found on the Falkland Islands and 10% on South Georgia. Background image on front and back cover: Shoal of fish (Charles Sheppard/Warwick -

Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11Th to 31St July 2015
Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11th to 31st July 2015 Orange Fruit Dove by K. David Bishop Trip Report - RBT Southwest Pacific Islands 2015 2 Tour Leaders: K. David Bishop and David Hoddinott Trip Report compiled by Tour Leader: K. David Bishop Tour Summary Rockjumper’s inaugural tour of the islands of the Southwest Pacific kicked off in style with dinner at the Stamford Airport Hotel in Sydney, Australia. The following morning we were soon winging our way north and eastwards to the ancient Gondwanaland of New Caledonia. Upon arrival we then drove south along a road more reminiscent of Europe, passing through lush farmlands seemingly devoid of indigenous birds. Happily this was soon rectified; after settling into our Noumea hotel and a delicious luncheon, we set off to explore a small nature reserve established around an important patch of scrub and mangroves. Here we quickly cottoned on to our first endemic, the rather underwhelming Grey-eared Honeyeater, together with Nankeen Night Herons, a migrant Sacred Kingfisher, White-bellied Woodswallow, Fantailed Gerygone and the resident form of Rufous Whistler. As we were to discover throughout this tour, in areas of less than pristine habitat we encountered several Grey-eared Honeyeater by David Hoddinott introduced species including Common Waxbill. And so began a series of early starts which were to typify this tour, though today everyone was up with added alacrity as we were heading to the globally important Rivierre Bleu Reserve and the haunt of the incomparable Kagu. We drove 1.3 hours to the reserve, passing through a stark landscape before arriving at the appointed time to meet my friend Jean-Marc, the reserve’s ornithologist and senior ranger. -

The Muslim Maroons and the Bucra Massa in Jamaica
AS-SALAAMU-ALAIKUM: THE MUSLIM MAROONS AND THE BUCRA MASSA IN JAMAICA ©Sultana Afroz Introduction As eight centuries of glorious Muslim rule folded in Andalusia Spain in 1492, Islam unfolded itself in the West Indian islands with the Andalusian Muslim mariners who piloted Columbus discovery entourage through the rough waters of the Atlantic into the Caribbean. Schooled in Atlantic navigation to discover and to dominate the sea routes for centuries, the mission for the Muslim mariners was to find the eternal peace of Islam as they left al-Andalus/Muslim Spain in a state of ‘empty husks’ and a land synonym for intellectual and moral desolation in the hands of Christendom Spain. The Islamic faith made its advent into Jamaica in1494 as these Muslim mariners on their second voyage with Columbus set their feet on the peaceful West Indian island adorned with wooded mountains, waterfalls, sandy beaches and blue seas. The seed of Islam sown by the Mu’minun (the Believers of the Islamic faith) from al-Andalus gradually propagated through the enslaved African Muslims from West Africa brought to serve the plantation system in Jamaica. Their struggle or resistance (jihad) against the slave system often in the form of flight or run away (hijra) from the plantations led many of them to form their own community (ummah), known as Maroon communities, a feature then common in the New World plantation economy.1 Isolationism and lack of Islamic learning made Islam oblivion in the Maroon societies, while the enslaved African Muslims on the plantations saw their faith being eclipsed and subdued by the slave institution, the metropolitan powers and the various Christian churches with their draconian laws. -

Australia's Biodiversity and Climate Change
Australia’s Biodiversity and Climate Change A strategic assessment of the vulnerability of Australia’s biodiversity to climate change A report to the Natural Resource Management Ministerial Council commissioned by the Australian Government. Prepared by the Biodiversity and Climate Change Expert Advisory Group: Will Steffen, Andrew A Burbidge, Lesley Hughes, Roger Kitching, David Lindenmayer, Warren Musgrave, Mark Stafford Smith and Patricia A Werner © Commonwealth of Australia 2009 ISBN 978-1-921298-67-7 Published in pre-publication form as a non-printable PDF at www.climatechange.gov.au by the Department of Climate Change. It will be published in hard copy by CSIRO publishing. For more information please email [email protected] This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction and rights should be addressed to the: Commonwealth Copyright Administration Attorney-General's Department 3-5 National Circuit BARTON ACT 2600 Email: [email protected] Or online at: http://www.ag.gov.au Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Climate Change and Water and the Minister for the Environment, Heritage and the Arts. Citation The book should be cited as: Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M and Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change. -

ATOLL RESEARCH BULLETIN NO. 251 BIOGEOGRAPHY of the PUERTO RICAN BANK by Harold Heatwole, Richard Levins and Michael D. Byer
ATOLL RESEARCH BULLETIN NO. 251 BIOGEOGRAPHY OF THE PUERTO RICAN BANK by Harold Heatwole, Richard Levins and Michael D. Byer Issued by THE SMITHSONIAN INSTITUTION Washington, D. C., U.S.A. July 1981 VIRGIN ISLANDS CULEBRA PUERTO RlCO Fig. 1. Map of the Puerto Rican Island Shelf. Rectangles A - E indicate boundaries of maps presented in more detail in Appendix I. 1. Cayo Santiago, 2. Cayo Batata, 3. Cayo de Afuera, 4. Cayo de Tierra, 5. Cardona Key, 6. Protestant Key, 7. Green Key (st. ~roix), 8. Caiia Azul ATOLL RESEARCH BULLETIN 251 ERRATUM The following caption should be inserted for figure 7: Fig. 7. Temperature in and near a small clump of vegetation on Cayo Ahogado. Dots: 5 cm deep in soil under clump. Circles: 1 cm deep in soil under clump. Triangles: Soil surface under clump. Squares: Surface of vegetation. X's: Air at center of clump. Broken line indicates intervals of more than one hour between measurements. BIOGEOGRAPHY OF THE PUERTO RICAN BANK by Harold Heatwolel, Richard Levins2 and Michael D. Byer3 INTRODUCTION There has been a recent surge of interest in the biogeography of archipelagoes owing to a reinterpretation of classical concepts of evolution of insular populations, factors controlling numbers of species on islands, and the dynamics of inter-island dispersal. The literature on these subjects is rapidly accumulating; general reviews are presented by Mayr (1963) , and Baker and Stebbins (1965) . Carlquist (1965, 1974), Preston (1962 a, b), ~ac~rthurand Wilson (1963, 1967) , MacArthur et al. (1973) , Hamilton and Rubinoff (1963, 1967), Hamilton et al. (1963) , Crowell (19641, Johnson (1975) , Whitehead and Jones (1969), Simberloff (1969, 19701, Simberloff and Wilson (1969), Wilson and Taylor (19671, Carson (1970), Heatwole and Levins (1973) , Abbott (1974) , Johnson and Raven (1973) and Lynch and Johnson (1974), have provided major impetuses through theoretical and/ or general papers on numbers of species on islands and the dynamics of insular biogeography and evolution. -

Bird Records from the Virgin Islands'', Published in the Journal of the Depar
SUPPLEMENTARY ACCOUNT OF THE BIRDS OF THE VIRGIN ISLANDS , INCLUDING CULEBRA AND ADJACENT ISLETS PERTAINING TO PUERTO RICO, WITH NOTES ON THEIR FOOD HABITS By S TUART T. DANFORTH Professor of Zoology and Entomology, College of Agriculture and Mechanics Arts. INTRODU CTION This paper is intended as a supp lement to my "B ird Records from the Virgin Islands'', published in the Journal of the Depart ment of Agriculture of Por to Rico, Vol. XIV, no. 3 (July, 1930). Since that pap er was published it has been my privi lege to continue ornithologica l observations in some of the islands previously visited, and to extend my investigations to a number of additiona l island-;, some of which had not previou sly been visited by an ornithologist . Reports on thr ee small but import ant collections of birds from St. Croix generously presented by Mr. Harry A. Beatty of that island are also included in this paper. IT INE RARIES In 1931 a short visit was made to St. Thomas and St . Croix en route to other islands on Ju ne 16, and on the return tri p the period from August 12 to 15 was spent on St. Thomas. During this short stay paroquets were obtained. In 1933 and 1934, with Mr. Angel Biaggi as assistant, a short collecting tr ip was made to St . Thomas and some of the Bri tish Virg in Islands. We arrived in St . Thomas very early in the morning of December 23, and worked there unti l the afternoon of December 27, when we went by motor laun ch to Tortola, where we stayed for a week, except for a half day visit which we made on December 28 to Beef Island , and the day of Janu ary 1, which we spent on Jost van Dyke. -

Protected Areas by Management 9
Unted States p Forest Department a Service DRNA of Agriculture g P r o t e c t e d N a t u r a l A r e a s o f P u e r to R i c o K E E P I N G C O M M ON S P E C I E S C O M M O N PRGAP ANALYSIS PROJECT William A. Gould, Maya Quiñones, Mariano Solórzano, Waldemar Alcobas, and Caryl Alarcón IITF GIS and Remote Sensing Lab A center for tropical landscape analysis U.S. Department of Agriculture, Forest Service, International Institute of Tropical Forestry . o c 67°30'0"W 67°20'0"W 67°10'0"W 67°0'0"W 66°50'0"W 66°40'0"W 66°30'0"W 66°20'0"W 66°10'0"W 66°0'0"W 65°50'0"W 65°40'0"W 65°30'0"W 65°20'0"W i R o t rotection of natural areas is essential to conserving biodiversity and r e u P maintaining ecosystem services. Benefits and services provided by natural United , Protected areas by management 9 States 1 areas are complex, interwoven, life-sustaining, and necessary for a healthy A t l a n t i c O c e a n 1 1 - 6 environment and a sustainable future (Daily et al. 1997). They include 2 9 0 clean water and air, sustainable wildlife populations and habitats, stable slopes, The Bahamas 0 P ccccccc R P productive soils, genetic reservoirs, recreational opportunities, and spiritual refugia.