The Red-Tailed Tropicbird on Kure Atoll
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 1. (Online Supplementary Material) Species, Gliding Strategies
Appendix 1. (Online Supplementary Material) Species, gliding strategies, species distributions, geographic range sizes, habitat, and egg buoyancy characteristics used for concentrated changes tests. Species Gliding strategy Species distribution (reference #) Geographic range size Habitat (reference #) Egg buoyancy (reference #) Cheilopogon abei (Parin, 1996) 4 wings Indian, Indo-Pacific (1) 2 or more ocean basins meroepipelagic (1) Buoyant (2) Cheilopogon atrisignis (Jenkins, 1903) 4 wings Indian, Pacific (1) 2 or more ocean basins meroepipelgic (3) Buoyant (4) Cheilopogon cyanopterus (Valenciennes, 1847) 4 wings Atlantic, Indo-Pacific (2) 2 or more ocean basins meroepipelgic (3) Non-Buoyant (5) Cheilopogon dorsomacula (Fowler, 1944) 4 wings Pacific (1) within 1 ocean basin holoepipelagic (1) Buoyant (2) Cheilopogon exsiliens (Linnaeus, 1771) 4 wings Atlantic (2) within 1 ocean basin holoepipelagic (3) Buoyant (2,5) Cheilopogon furcatus (Mitchill, 1815) 4 wings Atlantic, Indian, Pacific (6) 2 or more ocean basins holoepipelagic (3) Non-Buoyant (5) Cheilopogon melanurus (Valenciennes, 1847) 4 wings Atlantic (7) within 1 ocean basin meroepipelagic (7) Non-Buoyant (5,8) Cheilopogon pinnatibarbatus (californicus) (Cooper, 1863) 4 wings eastern tropical Pacific (9) within 1 ocean basin meroepipelgic (3) Non-Buoyant (10) Cheilopogon spilonotopterus (Bleeker, 1865) 4 wings Indian and Pacific (1) 2 or more ocean basins meroepipelgic (3) Buoyant (4) Cheilopogon xenopterus (Gilbert, 1890) 4 wings eastern tropical Pacific (11) within 1 ocean basin -
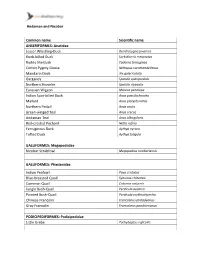
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Koa'e 'Ula Or Red-Tailed Tropicbird
Seabirds Koa‘e ‘ula or Red-tailed Tropicbird Phaethon rubricauda SPECIES STATUS: Photo: DOFAW State recognized as Indigenous NatureServe Heritage Ranking G4/G5 – Apparently secure/Secure North American Waterbird Conservation Plan – Moderate concern Regional Seabird Conservation Plan - USFWS 2005 SPECIES INFORMATION: The koa‘e ‘ula or red-tailed tropicbird is a showy, white seabird (Family: Phaethontidae) related to boobies and frigatebirds. Four koa‘e ‘ula (red-tailed tropicbird) subspecies are recognized, and one (P. r. roseotincta) breeds in Hawai‘i. Adult males and females are mostly white, although sometimes with pale pinkish wash, except for partial black eye ring and short eye line, black flanks, and black shafts of outer primaries; both sexes have long, narrow, tail feathers with red shafts. Large reddish orange bill with black tip; legs and feet are very small. Flight is characterized by strong flapping interspersed with gliding; koa‘e ‘ula (red-tailed tropicbird) are capable of flying long distances. Koa‘e ‘ula (red-tailed tropicbird) usually forage alone, but occasional with other species, most often far from land; often will follow ships. Koa‘e ‘ula (red-tailed tropicbird) captures prey by plunge diving. In Hawai‘i, diet is mainly comprised of flyingfish, but also takes squid, mackerel scads, dolphinfish, truncated sunfish, and ballonfish. Koa‘e ‘ula (red-tailed tropicbird) breed in colonies and pairs remain together for years. At the beginning of the breeding season, pairs engage in complex aerial displays. Nests are placed on the ground, and generally are a simple scrape lined with some vegetation. In Hawai‘i, breeding can occur throughout the year, but most nests are active between February and June. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Predator-Driven Macroevolution in Flyingfishes Inferred from Behavioural Studies 59
Predator-driven macroevolution in flyingfishes inferred from behavioural studies 59 Predator-driven macroevolution in flyingfishes inferred from behavioural studies: historical controversies and a hypothesis U. Kutschera Abstract Flyingfishes (Exocoetidae) are unique oceanic animals that use their tail and their large, wing-like pectoral fins to launch themselves out of the water and glide through the air. Independent observations document that flyingfishes use their gliding ability to escape from aquatic predators such as dolphins (marine mammals). The fossil record of flyingfishes is very poor. Nevertheless, the evolution of gliding among flyingfishes and their allies (Beloniformes) was analysed and reconstructed by the ethologist Konrad Lorenz (1903 – 1989) and other zoologists. In this article I review the comparative method in evolutionary biology, describe historical controversies concerning the biology and systematics of flyingfishes and present a hypothesis on the phylogenetic development of gliding among these marine vertebrates. This integrative model is based on behavioural studies and has been corroborated by molecular data (evolutionary trees derived from DNA sequences). Introduction Since the publication of Darwin´s classical book (1872, 1st ed. 1859), evolutionary biology has relied primarily upon comparative studies of extant organisms (animals, plants), supplemented whenever possible by information obtained from the fossil record. This interaction between neontological and palaeontological research has greatly enriched our knowledge of the evolutionary history (phylogeny) of a variety of macro- organisms, notably hard-shelled marine invertebrates (molluscs etc.) and vertebrates, for which thousands of well-preserved fossils have been described. Such comparative studies have become considerably more significant with the development of molecular methods for reconstructing DNA-sequence-based phylogenies and with the increased rigour with which the comparative method has been applied. -

2019 ABA Bird of the Year on the Biology, Field Identification, and General Coolness of the Red-Billed Tropicbird, Phaethon Aethereus
RED-BILLED TROPICBIRD | 2019 BIRD OF THE YEAR 2019 ABA Bird of the Year On the biology, field identification, and general coolness of the Red-billed Tropicbird, Phaethon aethereus IOANA SERITAN Berkeley, California [email protected] PETER PYLE San Francisco, California [email protected] 20 BIRDING | FEBRUARY 2019 he Red-billed Tropicbird is one of of the continental U.S. Read on to learn more Red-billed Tropicbird is joined by the White- three tropicbird species, all of which about tropicbirds in general and Red-billed tailed and Red-tailed tropicbirds to make up Tcan be found in the ABA Area with a Tropicbirds specifically—how to identify the monotypic family Phaethontidae within bit of legwork. Tropicbirds are a fun challenge them, where to find them, and why they are no the order Phaethontiformes. The latest ABA to find, a beauty to look at, and an interesting longer grouped with pelicans. Checklist, updated in December 2018, lists the evolutionary dilemma to consider. You may be Let’s start with some general context on White-tailed Tropicbird as Code 2 (regularly lucky enough to see a pair engaging in court- the tropicbird family. Tropicbirds are pelagic, occurring but range-restricted), while Red- ship display at a breeding site, perhaps meaning “open ocean,” birds that look kind of tailed and Red-billed are both Code 3 (rare in Hawaii, or you may be graced with like glorified terns. Their most famous phys- but annual); before Hawaii was added to the a sighting of a vagrant along ical features are the long tail plumes they ABA Area in late 2016, White-tailed was Code the East or West coast grow as adults. -

Diet of Elagatis Bipinnulata (Guoy & Gaimard, 1824) in Côte D’Ivoire (Gulf of Guinea)
European Scientific Journal January 2019 edition Vol.15, No.3 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 Diet of Elagatis bipinnulata (Guoy & Gaimard, 1824) in Côte d’Ivoire (Gulf of Guinea) Assan N’dri Florentine, Laboratoire de Biologie et de Cytologie Animales UFR des Sciences de la Nature, Université Nangui Abrogoua, Côte d’Ivoire Diaha N’guessan Constance, Amande Monin Justin, Département des Ressources Aquatiques Vivantes Centre de Recherches Océanologiques, Côte d’Ivoire Angui Kouamé Jean Paul, N’guessan Yao, Edoukou Abekan, N’da Konan, Laboratoire de Biologie et de Cytologie Animales UFR des Sciences de la Nature, Université Nangui Abrogoua, Côte d’Ivoire Guillou Aurélie Marie, MARBEC, Univ Montpellier, CNRS, Ifremer, IRD, Sète, France; Centre de Recherche Océanologiques, Côte d’Ivoire Doi: 10.19044/esj.2019.v15n3p131 URL:http://dx.doi.org/10.19044/esj.2019.v15n3p131 Abstract The rainbow runner, Elagatis bipinnulata, which belongs to the Carangidae family, is regularly encountered in the landing of artisanal fishing in Abobo-Doumé (Republic of Côte d’Ivoire). The object of this work is to study the diet of Elagatis bipinnulata according to the marine seasons and the size of the various specimens (immature and mature). About 900 fish measuring between 43 and 93 cm (fork length - FL) were examined from September 2015 to August 2017. On the 900 examined stomachs, 541 were empty, which is giving a vacuity coefficient of 60.11%. A feeding index (IRI: index of relative importance of food item) combining three methods percentage occurrence, numerical method and weight method) was used. The identification of the items found in the stomach contents revealed that the principals food items were fish (%IRI=48.85) and crustaceans (%IRI=45.78). -

Koa'e Kea Or White-Tailed Tropicbird
Seabirds Koa‘e kea or White-tailed Tropicbird Phaethon lepturus SPECIES STATUS: State recognized as Indigenous NatureServe Heritage Ranking G5 - Secure North American Waterbird Conservation Plan – Photo: Eric VanderWerf High concern Regional Seabird Conservation Plan - USFWS 2005 SPECIES INFORMATION: The koa‘e kea or white-tailed tropicbird is a showy, white seabird (Family: Phaethontidae), related to boobies and frigatebirds. Six koa‘e kea (white-tailed tropicbird) subspecies are recognized; only one (P. l. dorothea) breeds in Hawai‘i. Adult male and females are mostly white, although sometimes with pale pinkish wash, except for a narrow black eye patch, black streak on upper wings, and black on the leading edge of the outer primaries; both sexes have long, narrow, white central tail feathers. Large yellow-green bill; legs and feet are very small. Flight is characterized by rapid wing beats, interspersed with brief periods of gliding. Koa‘e kea (white-tailed tropicbird) usually forage alone, but occasional with conspecifics, most often far from land; often will follow ships. Koa‘e kea (white-tailed tropicbird) captures prey by plunge diving from 15 to 20 meters (50 – 65 feet) above the water. Diet is poorly known, but includes flyingfish and is likely similar to koa‘e ula or red-tailed tropicbird (P. rubricauda). Koa‘e kea (white-tailed tropicbird) breed in colonies and pairs remain together for years. At the beginning of the breeding season, pairs engage in complex aerial displays. Nests are placed in hard to reach locations on cliffs as well as in caves and tree hollows; nests have little if any material. -

Trophic Structure and Food Resources of Epipelagic and Mesopelagic Fishes in the North Pacific Subtropical Gyre Ecosystem Inferred from Nitrogen Isotopic Compositions
LIMNOLOGY and Limnol. Oceanogr. 00, 2015, 00–00 OCEANOGRAPHY VC 2015 Association for the Sciences of Limnology and Oceanography doi: 10.1002/lno.10085 Trophic structure and food resources of epipelagic and mesopelagic fishes in the North Pacific Subtropical Gyre ecosystem inferred from nitrogen isotopic compositions C. Anela Choy,*1,2 Brian N. Popp,3 Cecelia C. S. Hannides,1,3 Jeffrey C. Drazen1 1Department of Oceanography, University of Hawaii at Manoa, Honolulu, Hawaii 2Present address: Monterey Bay Aquarium Research Institute, Moss Landing, California 3Department of Geology & Geophysics, University of Hawaii at Manoa, Honolulu, Hawaii Abstract We used bulk tissue d13C and d15N values and d15N values of individual amino acids (AA) to characterize the trophic structure of a pelagic fish assemblage from the North Pacific Subtropical Gyre (NPSG) ecosystem. We focus on energy flow between fishes inhabiting distinct epipelagic, mesopelagic, and upper bathypelagic habitats and on predatory fish foraging across and within these depth habitats. Trophic positions (TPs) esti- 15 mated from a combination of trophic and source AA d N values (TPTr-Src) spanned a narrow range of 0.7 TP for 10 species of large fishes, including tunas, billfishes, and gempylids (TPTr-Src 4.3-5.0). Similarly, 13 species 15 of small micronekton fishes encompassed a range of 1.2 TP (TPTr-Src 2.6-3.8). The d N values of three source 15 AAs were found to increase with increasing depth of capture across the 13 micronekton fish species (d NPhe 15 15 range 5 6.6&; d NGly range 5 13.4&; d NSer range 5 13.6&), indicating that some species from epipelagic, mesopelagic, and upper bathypelagic communities access distinct food resources, such as suspended particles.